There is a recent study out which looks at the potential for a chemical derived from mushrooms to slow the progression of one type of cancer in dogs. It has both strengths and weaknesses, and it is a nice example of both good preliminary, exploratory research and why we shouldn’t put too much confidence in the findings of such research.
Dorothy Cimino Brown and Jennifer Reetz. Single Agent Polysaccharopeptide DelaysMetastases and Improves Survival in Naturally Occurring Hemangiosarcoma. Evidence-Based Complementary and Alternative Medicine. Volume 2012, Article ID 384301, 8 pages. doi:10.1155/2012/384301
The goal of the study was to see if a Chinese herbal medicine product, ImYunity, containing a standardized amount of a chemical called polysaccharopeptide (PSP), had an effect on the progression of the malignant blood vessel cancer hemangiosarcoma (HSA) in dogs. The dogs were those diagnosed with HSA after having surgery to remove their spleens, which were bleeding due to the presence of HSA. Typically, dogs with HSA who have a splenectomy and no other treatment only live a couple of months, though of course there is enough variation that the life expectancy of any individual patient cannot be accurately predicted. Given chemotherapy, these patients tend to live quite a bit longer, however most still do not survive to a year, and all will eventually die from metastatic HSA. Given this poor prognosis even with standard therapy, it is certainly reasonable to investigate alternative approaches.
PSP is believed to be the active compound in some mushrooms used for medicinal purposes, and there is a moderate amount of in vitro and laboratory animal research to suggest the compound may have some effects on the immune system and on cancer cells. I agree with the authors of this paper point out, however, when they say that “as with all other preclinical data, whether these reported findings of suppression of cell proliferation and induction of apoptosis in malignant cells from murine models have relevance in patient care is uncertain.”
Methodologically, the study was of mixed quality. There were only 15 subjects, which is a very small number, and this can lead to random individual variations having a significant effect on any differences seen between groups of subjects. The dogs were selected on the basis of having been diagnosed with HSA and having had their spleens removed. Some had metastases elsewhere in their abdomen at the time of diagnosis and others did not. The dogs were randomly assigned to three different dosage levels of PSP. The investigators and owners were blinded to which dose each dog was getting. However, there was no control group receiving either a placebo or standard therapy.
The two main outcomes studied were time to progression of metastatic disease in the abdomen and survival. While survival is a fairly clear outcome, it is influenced by factors other than the just the progression of the disease since most dogs with cancer are euthanized at their owners’ request. The factors that lead an owner to be ready to do this are many and varied, and not all hinge on the stage of disease in the pet. Still, this is a useful measure in the real world since presumably an effective therapy which slows the progress of disease will be an important factor in determining how long it is before an owner feels it is time to let the pet go.
The second outcome measure is a little less clearly described. An abdominal ultrasound was done on all patients at the time of their enrollment in the study and them monthly thereafter. It isn’t perfectly clear from the report, but it appears that the radiologists performing these examinations were unaware of the treatment each dog was receiving. According to the paper, “Based on this comparison, the board certified radiologist made the determination of progression of metastatic disease.” It’s also not entirely clear what criteria were used to determine progression. How many new lesions or how much growth in established lesions counted as “progression,” and how standardized and consistent was this determination between different radiologists? Such a subjective measure can complicate interpretation of the effect of a therapy.
The results were relatively unimpressive, though some could be consistent with a therapeutic benefit. There was no difference in the median survival among the three groups. However, dogs in the middle and high dose groups lived longer than has previously been reported for dogs with HAS treated only with surgery. This could be consistent with the theory that PSP had some effect on survival.
However, in the absence of an untreated control group, it is at least as likely that this group of dogs differed from those for which survival has previously been reported in some way other than the use of PSP. For example, dogs treated with surgery and not chemotherapy are typically not given any other treatment, and they are certainly not extensively evaluated monthly at a university veterinary hospital. Perhaps the owners who were willing to be a part of this trial were likely to take longer to choose euthanasia than those owners not interested in having their dogs take part for psychological reasons. Or perhaps they generally took better care of their dogs than owners not interested in participating. And it is well know that, for a variety of reasons, people who participate in a research study have better outcomes than those who don’t even if the participants are only getting a placebo, for a variety of reasons. So this result cannot reliable establish that the PSP had any effect on how long these dogs lived.
The other outcome measure, time to progression of abdominal metastases, only showed a statistically significant difference between the high-dose group and the low-dose group. There was no detectable difference between the middle group and either of the others. And the p-value, which establishes how likely this difference was to be due to chance (though not that the difference was due to the treatment), was 0.046. The usual cutoff value for significance is 0.05, so this value was only marginally significant. The difference might be due to an effect of treatment, but it might also be due to chance, especially given the small number of dogs in the trial and the lack of any control groups.
A reasonable interpretation of these results is that this small pilot study found a marginal difference between progression in the high dose group and the low dose, and this could suggest an effect of the drug or could be due to chance, measurement variability, bias, or some other factor. And the survival of the high dose group, which was roughly 6 ½ months compared to the previously reported 3 months for dogs treated with surgery alone (and 6-9 months with chemotherapy), could represent a small benefit from the PSP. Or it could simply be due to random variation or some other, undetected difference between this group of dogs and those studied previously.
Not surprisingly, the authors take a somewhat more positive position on the significance of the results.
While it is necessary to now document more robust and statistically significant differences in an appropriately powered definitive placebo controlled study, it is encouraging that clear patterns and an obvious dose choice emerged from the pilot program.
Based on this data, one could hypothesize that PSP has the potential to have effects on survival similar to that which is seen with standard of care chemotherapy.
Proving, in a biologically aggressive animal model, that PSP delivers antitumor and survival effects in a magnitude similar to that which is seen in standard chemotherapy could have significant implications for shifts in standard of care from current cytotoxic therapies to complementary compounds, such as PSP, that have little to no negative documented effects on normal cells. Most importantly, for those cancer patients throughout the world for whom advanced treatments and cytotoxic therapies are not accessible, CAM, such as PSP as a single agent, could offer benefits to survival and quality of life that are not yet imagined for those populations.
One could hypothesize that PSP is as good as standard chemotherapy for this disease, but that’s only a guess, and this study does little to support that hypothesis. The authors are correct to acknowledge that additional work is needed to demonstrate this is actually true, but they strike a tone that is just a touch more optimistic than these data would seem to justify. Such is inevitable when a researcher has a lot invested in their hypothesis, and it is understandable that the authors would try to put as positive a spin on the results as possible.
However, the suggestion that this therapy, or other alternative therapies, might potentially replace chemotherapy for cancer care is thoroughly unwarranted. For one thing, it has been shown repeatedly than avoiding conventional care in favor of alternative therapies, or even adding these therapies to conventional care, more commonly worsens outcome and quality of life. (see the studies below for a few examples). So it would take a much stronger result than that shown in this paper to even begin to justify the suggestion that alternative therapies could ever be considered a replacement for conventional care.
And the authors also seem to suggest one advantage of PSP as a cancer therapy is the absence of any side effects. No truly effective therapy for a serious disease has ever been found that is free of side effects, so this is an extraordinary hypothesis, which will require extraordinary evidence to prove. Given how unlikely the idea is, it deserves great skepticism and it is ethically questionable to put it forward as an appropriate expectation.
Finally, the authors justify their study partly on the principle that many people cannot afford conventional cancer therapy and that cheaper, possibly safer alternatives should be sought.
A large percentage of [cancer patients] will live in countries that lack the resources for cancer control. The dramatic technological changes that will continue in surgery, radiotherapy, and chemotherapy will lead to increased cure rates; however, these anticipated advances come at a price usually beyond the means of most cancer patients. It is imperative that affordable complementary and alternative treatment strategies, that could considerably reduce the global disease burden at manageable costs, are developed.
There is clear evidence that health and longevity have improved since the advent of a science-based approach to health, and that the benefits of scientific medicine have been greatest in countries which are affluent enough for people to have access to this kind of care (Fig. 1). In poorer countries, people often are forced to rely on traditional folk medicine practices that have not been scientifically validated, and they frequently have poorer health. So there is no question that there is a need to provide better healthcare to people in these countries. There is not, however, any justification for suggesting that alternative medicine, even if rigorously investigated scientifically, will meet this need.
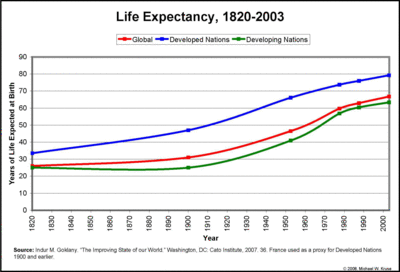
This figure shows how life expectancy, one measure of health, has increased as the scientific approac to health has replaced traditional medicine, and how lack of access to scientific medicine is associated with lower life expectancy.
For one thing, the reason conventional care is so expensive is largely because tremendous resources are needed to properly develop and research therapies to ensure they are safe and effective. Alternative therapies are largely cheaper because this rigorous scientific study has not been carried out and such therapies are not subject to meaningful regulation requiring evidence of safety and efficacy or verifiable quality control in production and distribution. There is no reason to suppose that if alternative therapies were subject to the same standards of evidence and the same regulatory oversight as conventional medicine that they would be any cheaper.
And it strikes me as ethically questionable to suggest that the proper answer to the healthcare needs of people without access to the best scientific medicine available should be met by putting resources into developing alternative medical therapies for them. These resources would likely be better spent reducing the cost and increasing the availability of therapies already established to be effective. It is perfectly fine to look for additional or better treatments in alternative medicine (subject to reasonable use of plausibility and pre-clinical research to target limited resources efficiently), but the suggestion that such therapies can or should replace conventional care for economically disadvantaged people is not reasonable. There is no good evidence these therapies will be as safe or effective as conventional care or cheaper, so this is a weak justification for research efforts directed at alternative interventions.
Bottom Line
This paper provides an intriguing suggestion that there might be benefit to mushroom-derived PSP for patients with hemangiosarcoma. It is by no means adequate in size or design to demonstrate this to be true, and the results are actually quite weak and as likely to be due to chance or bias as to an actual effect of PSP on this cancer. However, further research into this therapy is certainly justified.
The suggestion that alternative medicine is likely to provide therapies that are as effective as conventional therapy with no side effects is implausible and contradicted by existing evidence. The idea that the proper response to the inadequate availability of proven cancer therapies for economically disadvantaged people is to put resources into investigating alternative medicine and developing a separate set of therapies for these people is an ethically dubious proposition. While investigating such therapies may be appropriate given sufficient evidence to suggest they will prove useful, it would be more appropriate to address healthcare inequities directly rather than justifying such investigation with the goal of developing separate treatments for people with different socioeconomic status.
- Han E, Johnson N, Delamelena T, Glissmeyer M, Steinbock K. Alternative therapy used as primary treatment for breast cancer negatively impacts outcomes. Ann Surg Oncol 2011;Jan 12 [Epub ahead of print].
- John A. Chabot, Wei-Yann Tsai, Robert L. Fine, Chunxia Chen, Carolyn K. Kumah, Karen A. Antman, and Victor R. Grann. Pancreatic Proteolytic Enzyme Therapy Compared with Gemcitabbine-based Chemotherapy for the Treatment of Pancreatic Cancer. Journal of Clinical Oncology. 2010;28(12):2058-63.
- Kurian Joseph, Sebastian Vrouwe, Anmmd Kamruzzaman, Ali Balbaid, David Fenton, Richard Berendt, Edward Yu and Patricia Tai. Outcome analysis of breast cancer patients who declined evidence-based treatment.World Journal of Surgical Oncology 2012, 10:118.
- Risberg T, Vickers A, Bremnes RM, Wist EA, Kaasa S, Cassileth BR. Does use of alternative medicine predict survival from cancer? Eur J Cancer. 2003 Feb;39(3):372-7.










This commentary is a wonderful and balanced assessment of a potential new therapy. The pros and cons described are typical of many veterinary studies, traditional or alternative. As a veterinary oncologist, we are always looking for new and better ways to treat cancer, especially aggressive cancers that are resistant to many of our current treatments. At The Veterinary Cancer Center, our practice in Connecticut, we always try to balance statistics with hope—as animals are not statistics and stats are great for evaluating groups, not individuals. This blog does a wonderful job of giving us hope while keeping us grounded in science.
Thanks, that’s certainly what I hope to do!
Someone just mentioned this to me for my dog’s hemangiosarcoma. Glad I have a logical resource to assess reality.
Has anything changed since 2012 ?
Sorry, I don’t see any additional research on this one since the 2012 paper.
There is additional research being done but it will not be complete for quite some time. The authors are now doing a large scale study that includes chemo & I’mYunity, just I’mYunity and just chemo. Unfortunately, it’s findings will be too late for my dog.
Yes, that’s frustrating. Science gives us the best, most reliable answers, but it does take time and the answers are not always ready when we need them. 🙁
Good luck.
Can I ask if you have any information about medical cannabis. I know someone whose dog has survived for 2 years on RSO 1:5 CBD/THC ratio. I know another person whose dog did not do well on the more readily available hemp CBD w low THC. Not trying to get my dog high and not even sure where I will be able to get this tincture.. but if your dog is going to die anyway and chemo is not that helpful for the cancer in question, you might as well pull out the stops. (Although that can not always be the case. Steve Jobs would have been well to go the traditional route with his supposedly curable cancer) Thanks for your help, even though it appears I may have to learn from experience. :/