I recently gave a lecture at the Western Veterinary Conference called “What You Know that Ain’t Necessarily So.” The purpose of this was to take some common or controversial beliefs and practices in veterinary medicine and discuss the scientific evidence pertaining to these. This was not intended as a definitive, “final word” on these subjects, but as an illustration of how weak and problematic the evidence often is even behind widely held beliefs. In some cases, these practices or ideas may actually be valid, but without good quality scientific evidence, we should always be cautious and skeptical about them.
Eventually, I will post recordings of the presentations themselves, but for now I am posting a summary of each topic.
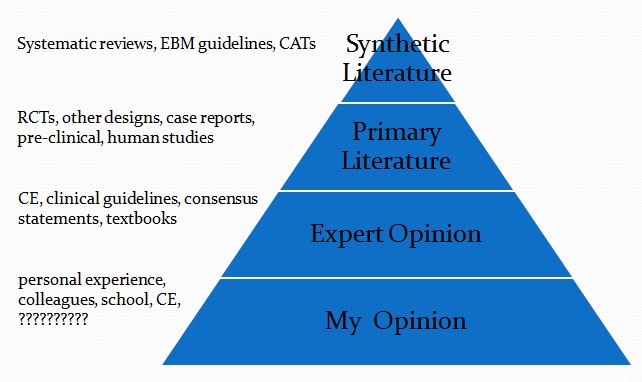
Each starts with a focused clinical question using the PICO format.
P– Patient, Problem Define clearly the patient in terms of signalment, health status, and other factors relevant to the treatment, diagnostic test, or other intervention you are considering. Also clearly and narrowly define the problem and any relevant comorbidities. This is a routine part of good clinical practice and so does not represent “extra work” when employed as part of the EBVM process.
I– Intervention Be specific about what you are considering doing, what test, drug, procedure, or other intervention you need information about.
C– Comparator What might you do instead of the intervention you are considering? Nothing is done in isolation, and the value of most of our interventions can only be measured relative to the alternatives. Always remember that educating the client, rather than selling a product or procedure, should often be considered as an alternative to any intervention you are contemplating.
O– Outcome What is the goal of doing something? What, in particular, does the client wish to accomplish. Being clear and explicit, with yourself and the client, about what you are trying to achieve (cure, extended life, improved performance, decreased discomfort, etc.) is essentially in evidence-based practice.
This is then followed by a summary of the evidence available at each of the levels in the following pyramid (which is a pragmatic reinterpretation of the classical pyramid of evidence that is a bit more useful for general practice veterinarians).
Finally, I list the Bottom Line, which is my interpretation of the evidence.
Glucosamine for Dogs with Arthritis
- Clinical question
P– Dogs with naturally occurring arthritis
I– oral glucosamine
C– NSAID, nothing
O– Reduced pain, lameness
2. Synthetic Veterinary Literature
a. Three systematic reviews:
the global strength of evidence of efficacy was low…In addition, results were contradictory in the 2 studies conducted in dogs. (Vandeweerd et al., 2012)
Low quality & quantity of evidence, no overall recommendation. (Sanderson et al., 2009)
One study included, good quality, no benefit (Aragon, Hofmeister, & Budsberg, 2007)
b. Three critically appraised topics (include same 2 studies as systematic reviews)
Best Bets for Vets Nutraceuticals versus carprofen in dogs with osteoarthritis
Carprofen is superior to glucosamine/chondroitin supplements in reducing the clinical signs of osteoarthritis (McCarthy et al. 2007). Glucosamine and chondroitin supplement efficacy cannot be commented on, as there was no placebo group or there was no comparison made with the placebo group in the studies.
Despite some evidence that a combination of glucosamine hydrochloride and chondroitin sulfate nutraceuticals improves symptoms associated with joint disease in dogs and cats, strong clinical evidence of efficacy is lacking, and these compounds are understudied.
What’s the Evidence? Glucosamine for osteoarthritis in dogs 2 studies, mixed results, better quality study found no benefit, carprofen better (McKenzie, 2010)
2. Primary Veterinary Literature
Already reviewed in synthetic literature
3. Human Literature
a. Systematic Reviews (dozens, these are just a few representative ones)
[Glucosamine] is ineffective for pain reduction in patients with knee OA. GS may have function-modifying effects in patients with knee OA when administered for more than 6 months. However, it showed no pain-reduction benefits after 6 months of therapy. (Wu, 2013)
Significant improvement in pain and functional indices and a decrease in the loss of joint space width were demonstrated in some but not all studies…The safety of these nutraceuticals has been demonstrated across all of the reviewed trials, and there were no significant issues with tolerance…An overall recommendation to use nutraceuticals in the treatment of all patients with OA is not strongly supported by the available data. (Ragle, 2012)
Compared with placebo, glucosamine, chondroitin, and their combination do not reduce joint pain or have an impact on narrowing of joint space. Health authorities and health insurers should not cover the costs of these preparations, and new prescriptions to patients who have not received treatment should be discouraged. (Wandel, 2010)
Pooled results from studies using a non-Rotta preparation or adequate allocation concealment failed to show benefit in pain and WOMAC function while those studies evaluating the Rotta preparation showed that glucosamine was superior to placebo in the treatment of pain and functional impairment resulting from symptomatic OA. (Towheed, 2005)
Most of the observed heterogeneity in glucosamine trials is explained by brand…Large inconsistency was found though. Low risk of bias trials, using the Rottapharm|Madaus product, revealed a small effect size. (Eriksen, 2014)
b. Clinical Practice Guidelines
We cannot recommend using glucosamine and chondroitin for patients with symptomatic osteoarthritis of the knee…. At this time, both glucosamine and chondroitin sulfate have been extensively studied. Despite the availability of the literature, there is essentially no evidence that minimum clinically important outcomes have been achieved compared to placebo, whether evaluated alone or in combination. American Academy of Orthopedic Surgeons
We conditionally recommend that patients with OA should not use the following:
Chondroitin sulfate Glucosamine
American College of Rheumatology
Glucosamine and chondroitin were both found to be “not appropriate” for all patients when used for disease modification and “uncertain” for all patients when used for symptom relief. Osteoarthritis Research Society International
c. Primary Human Literature
Glucosamine/Arthritis Intervention Trial (GAIT)
Over 2 years, no treatment achieved a clinically important difference in WOMAC pain or function as compared with placebo…. Glucosamine and chondroitin sulfate alone or in combination did not reduce pain effectively in the overall group of patients with osteoarthritis of the knee. Exploratory analyses suggest that the combination of glucosamine and chondroitin sulfate may be effective in the subgroup of patients with moderate-to-severe knee pain.
At 2 years, no treatment achieved a predefined threshold of clinically important difference in JSW loss as compared with placebo.
Bottom Line-
- Almost certainly safe
- Basic science supports potential benefits
- Very limited research in dogs
- Weak and conflicting evidence
- Little reason to believe significant benefits
- Extensive human research
- Conflicting evidence
- Most likely little to no benefit
References
Aragon, C. L., Hofmeister, E. H., & Budsberg, S. C. (2007). Topics in Drug Therapy of treatments for osteoarthritis in dogs. Journal of the American Veterinary Medical Association, 230(4).
McKenzie, B. A. (2010). What Is the Evidence?? Glucosamine for osteoarthritis in dogs. Journal of the American Veterinary Medical Association, 237(12), 1382–1383.
Ragle, RL. et al. Nutraceuticals in the management of osteoarthritis : a critical review. Drugs Aging. 2012 Sep;29(9):717-31.
Sanderson, R. O., Beata, C., Flipo, R.-M., Genevois, J.-P., Macias, C., Tacke, S., … Innes, J. F. (2009). Systematic review of the management of canine osteoarthritis. The Veterinary Record, 164, 418–424. doi:10.1136/vr.164.14.418
Vandeweerd, J.-M., Vandeweerd, S., Gustin, C., Keesemaecker, G., Cambier, C., Clegg, P., … Gustin, P. (2012). Understanding Veterinary Practitioners’ Decision-Making Process: Implications for Veterinary Medical Education. Journal of Veterinary Medical Education, 39, 142–151. doi:10.3138/jvme.0911.098R1
Wu, D. et al. Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials. Int J Clin Pract. 2013










Hello,
Thank you for sharing your research.
I have an older dog with arthritis and we actually just started her on a higher quality GC and have actually noticed some improvements. I am a dietitian so research is very, very important to me and have been doing some investigating on the benefits of curcumin, boswellin, and bioperine for her arthritis but was wondering if you had any feedback on that combination supplement for dogs. I only seem to find isolated curcumin supplements or boswellin for dogs but not this combo and I want to ensure it’s safe for her. We currently give her boswellin but it doesn’t seem to be doing a whole lot. Any thoughts, please? Our current vet is very mainstream and isn’t able to help us much in this area.
I’m not aware of any controlled research on the combination. I have written about curcumin, and so far there isn’t much direct research evidence to show benefits. Some promising preliminary evidence exists, but nothing solid. There is far less evidence for Boswellia, so in general I’m not yet convinced that has much value.
Brennen ,
What do you think of this new study:https://www.ncbi.nlm.nih.gov/pubmed/28533290
?
This seems to show a pattern quite similar to other similar studies of nutraceutical for OA. All patients improve by a significant amount in the study, and the differences between the extent of improvement among treatment groups are much smaller than the effect of being in the study. This raises significant questions about how much real, meaningful impact the treatment had. For example, on a 100-point pain scale, the three groups showed the following improvement:
Placebo- 33.4 points
Chondroitin- 42.6 points
Celecoxib- 39.5 points
So the pain decreased an average of 40.5 points on a 100-point scale, but the differences between the groups were 6.1-9.2 points. That means the differences were pretty small compared to the overall improvement, suggesting just being in the trial contributed to most of the change. At most, the true underlying effect of the chondroitin would have been 9% better than nothing, which may be statistically significant but of questionable real-world significance.
Looked at another way, the minimum difference thought to have any clinical significance is 5 points, so technically a 9-point difference might be detectable by a patient. However, doing nothing except enrolling in the trial yielded 33-point difference, which is a LOT bigger! Perhaps just giving out placebos should be our first therapy for OA. 🙂
I also noticed that more people in the chondroitin group (39) and the placebo group (33) dropped out of the study than in the celecoxib group (27), which raises the question of whether subjects in those groups were more likley to drop out because they weren’t getting as much relief. And the number dropping out due to side effects from the drug was the same for chondroitin and celecoxib (8), which undermines the argument that chondroitin is better tolerated than NSAIDs.
It is possible that the chondroitin really did have as much effect and the celecoxib and that both were only barely more effective than placebo. If so, this seems more an indictment of the celecoxib than a validation of the use of chondroitin since NSAIDs usually perform better than this against placebo. In a meta-analysis, for example, celecoxib was on average 17% better than placebo (0.86 on a 5-point scale), which is almost three times the difference in this study (6.1% or 6.1 points on a 100-point scale). It makes one wonder about the accuracy of assessment or the effectiveness of the positive comparator inthis study.
I still struggle to reconcile the evidence with my experience with my older dog, who started on a supplement after diagnosis of mild OA and a short course of NSAIDs. It was two years before her condition worsened enough to need NSAIDs again. I understand this can be explained but I do wonder if future studies may find a benefit.
Are you aware of any studies that look at the impact of glucosamine and chondroitin on joint health and disease prevention, as opposed to treatment of OA? I wondered if there could be some benefit in slowing cartilage degeneration, but most studies I’m aware of tend to focus on treating OA. Aware there is much more complexity in this kind of longitudinal study, so I assume it’s unlikely, but thought you would know!
Lots of research in humans, not much in dogs. Overall, the dog research is unconvincing. The human research is mixed, but it’s been hard to find any consistent, meaningful benefits despite decades of research in thousands of people, which doesn’t seem cause for much optimism. Here are some of the most recent reviews:
Open Vet J. 2017; 7(1): 36–49.
Published online 2017 Feb 24. doi: 10.4314/ovj.v7i1.6
Glucosamine and chondroitin use in canines for osteoarthritis: A review
Angel Bhathal, Meredith Spryszak, Christopher Louizos, and Grace Frankel
Clin Exp Rheumatol. 2018 Jan 31. [Epub ahead of print]
Comparative effectiveness of glucosamine, chondroitin, acetaminophen or celecoxib for the treatment of knee and/or hip osteoarthritis: a network meta-analysis.
Zhu X1, Wu D1, Sang L2, Wang Y2, Shen Y3, Zhuang X2, Chu M2, Jiang L4.
Author information
Abstract
OBJECTIVES:
To compare the efficacies of oral glucosamine, chondroitin, the combination of glucosamine and chondroitin, acetaminophen and celecoxib on the treatment of knee and/or hip osteoarthritis.
METHODS:
We searched electronic databases including PubMed, Embase, and Cochrane Library and the reference lists of relevant articles published from inception to October 23, 2017. A Bayesian hierarchical random effects model was used to examine the overall effect size among mixed multiple interventions.
RESULTS:
We identified 61 randomised controlled trials of patients with knee and/or hip osteoarthritis. There was no obvious difference in the results between the traditional meta-analysis and the network meta-analysis. The network meta-analysis demonstrated that celecoxib was most likely the best option (SMD, -0.32 [95% CI, -0.38 to -0.25]) for pain, followed by the combination of glucosamine and chondroitin. For physical function, all interventions were significantly superior to oral placebo except for acetaminophen. In terms of stiffness, glucosamine(SMD, -0.36 [95% CI, -0.67 to -0.06]) and celecoxib (SMD, -0.29 [95% CI, -0.51 to -0.08]) were significantly better compared to placebo. In view of safety, compared to placebo only, celecoxib and acetaminophen presented significant differences.
CONCLUSIONS:
Given the effectiveness of these non-steroidal anti-inflammatory drugs and symptomatic slow-acting drugs, oral celecoxib is more effective than placebo on relieving pain and improving physical function, followed by the combination of glucosamine and chondroitin. Acetaminophen is likely the least efficacious intervention option. This information, accompanied by the tolerability and economic costs of the included treatments, would be conducive to making decisions for clinicians.
Br J Sports Med. 2018 Feb;52(3):167-175. doi: 10.1136/bjsports-2016-097333. Epub 2017 Oct 10.
Dietary supplements for treating osteoarthritis: a systematic review and meta-analysis.
Liu X1,2, Machado GC3, Eyles JP1,2,4, Ravi V1,2, Hunter DJ1,2.
Author information
Abstract
OBJECTIVE:
To investigate the efficacy and safety of dietary supplements for patients with osteoarthritis.
DESIGN:
An intervention systematic review with random effects meta-analysis and meta-regression.
DATA SOURCES:
MEDLINE, EMBASE, Cochrane Register of Controlled Trials, Allied and Complementary Medicine and Cumulative Index to Nursing and Allied Health Literature were searched from inception to April 2017.
STUDY ELIGIBILITY CRITERIA:
Randomised controlled trials comparing oral supplements with placebo for hand, hip or knee osteoarthritis.
RESULTS:
Of 20 supplements investigated in 69 eligible studies, 7 (collagen hydrolysate, passion fruit peel extract, Curcuma longa extract, Boswellia serrata extract, curcumin, pycnogenol and L-carnitine) demonstrated large (effect size >0.80) and clinically important effects for pain reduction at short term. Another six (undenatured type II collagen, avocado soybean unsaponifiables, methylsulfonylmethane, diacerein, glucosamine and chondroitin) revealed statistically significant improvements on pain, but were of unclear clinical importance. Only green-lipped mussel extract and undenatured type II collagen had clinically important effects on pain at medium term. No supplements were identified with clinically important effects on pain reduction at long term. Similar results were found for physical function. Chondroitin demonstrated statistically significant, but not clinically important structural improvement (effect size -0.30, -0.42 to -0.17). There were no differences between supplements and placebo for safety outcomes, except for diacerein. The Grading of Recommendations Assessment, Development and Evaluation suggested a wide range of quality evidence from very low to high.
CONCLUSIONS:
The overall analysis including all trials showed that supplements provided moderate and clinically meaningful treatment effects on pain and function in patients with hand, hip or knee osteoarthritis at short term, although the quality of evidence was very low. Some supplements with a limited number of studies and participants suggested large treatment effects, while widely used supplements such as glucosamine and chondroitin were either ineffective or showed small and arguably clinically unimportant treatment effects. Supplements had no clinically important effects on pain and function at medium-term and long-term follow-ups.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Ann Rheum Dis. 2017 Nov;76(11):1862-1869. doi: 10.1136/annrheumdis-2017-211149. Epub 2017 Jul 28.
Subgroup analyses of the effectiveness of oral glucosamine for knee and hip osteoarthritis: a systematic review and individual patient data meta-analysis from the OA trial bank.
Runhaar J1, Rozendaal RM1, van Middelkoop M1, Bijlsma HJW2, Doherty M3, Dziedzic KS4, Lohmander LS5, McAlindon T6, Zhang W3, Bierma Zeinstra S7.
Author information
Abstract
OBJECTIVE:
To evaluate the effectiveness of oral glucosamine in subgroups of people with hip or knee osteoarthritis (OA) based on baseline pain severity, body mass index (BMI), sex, structural abnormalities and presence of inflammation using individual patient data.
METHODS:
After a systematic search of the literature and clinical trial registries, all randomised controlled trials (RCTs) evaluating the effect of any oral glucosamine substance in patients with clinically or radiographically defined hip or knee OA were contacted. As a minimum, pain, age, sex and BMI at baseline and pain as an outcome measure needed to be assessed.
RESULTS:
Of 21 eligible studies, six (n=1663) shared their trial data with the OA Trial Bank. Five trials (all independent of industry, n=1625) compared glucosamine with placebo, representing 55% of the total number of participants in all published placebo-controlled RCTs. Glucosamine was no better than placebo for pain or function at short (3 months) and long-term (24 months) follow-up. Glucosamine was also no better than placebo among the predefined subgroups. Stratification for knee OA and type of glucosamine did not alter these results.
CONCLUSIONS:
Although proposed and debated for several years, open trial data are not widely made available for studies of glucosaminefor OA, especially those sponsored by industry. Currently, there is no good evidence to support the use of glucosamine for hip or knee OA and an absence of evidence to support specific consideration of glucosamine for any clinically relevant OA subgroup according to baseline pain severity, BMI, sex, structural abnormalities or presence of inflammation.
Int J Clin Pract. 2013 Jun;67(6):585-94. doi: 10.1111/ijcp.12115.
Efficacies of different preparations of glucosamine for the treatment of osteoarthritis: a meta-analysis of randomised, double-blind, placebo-controlled trials.
Wu D1, Huang Y, Gu Y, Fan W.
Author information
Abstract
OBJECTIVE:
To determine the efficacies of different preparations of glucosamine for the treatment of osteoarthritis (OA).
METHODS:
Systematic searches of the bibliographic databases Medline, Embase, the Cochrane Central Register of Controlled Trials and the Cochrane Database of Systematic Reviews for randomised, double-blind, placebo-controlled trials (RCTs) concerning glucosamine treatment of OA. Effect size (ES) was estimated using Cohen’s standardised mean difference. Consistency was evaluated via the I(2) index.
RESULTS:
Nineteen trials (3159 patients) contributed to the meta-analysis, revealing a large degree of inconsistency among the trials in terms of pain-reduction outcome: the combined ES in glucosamine sulphate (GS) trials was -0.22 [95% confidence intervals (CI) -0.48, 0.04], I(2) was 82.3%. The combined ES in glucosamine hydrochloride (GH) trials was -0.03 (95% CI -0.14, 0.08), with an absence of heterogeneity. No treatment ES was observed [-0.38 (95% CI -0.99, 0.23)] favouring GS in trials of less than 24 weeks duration and the I(2) remained high (I(2) = 88.5%). No significant treatment ES -0.09 (95% CI -0.21, 0.03) was observed in trials of more than 24 weeks duration compared with placebo, with a heterogeneity of zero. In terms of function-modifying outcomes, GS showed no significant effect on Lequesne Index reduction vs. placebo in trials of less than 24 weeks duration (ES -0.55 (95% CI -1.22, 0.11)) with a high degree of heterogeneity (I(2) = 92.9%). Pooling data from studies with durations of more than 24 weeks presented a significant combined ES of -0.36 (95% CI: -0.56, -0.17) with an absence of heterogeneity. No risk of publication bias could be detected using Egger test.
CONCLUSIONS:
GH is ineffective for pain reduction in patients with knee OA. GS may have function-modifying effects in patients with knee OA when administered for more than 6 months. However, it showed no pain-reduction benefits after 6 months of therapy.
Eur J Med Res. 2015 Mar 13;20:24. doi: 10.1186/s40001-015-0115-7.
Efficacy and safety of glucosamine, diacerein, and NSAIDs in osteoarthritis knee: a systematic review and network meta-analysis.
Kongtharvonskul J1, Anothaisintawee T2, McEvoy M3, Attia J4, Woratanarat P5, Thakkinstian A6.
Author information
Abstract
BACKGROUND:
To conduct a systematic review and network meta-analysis of randomized controlled trials (RCTs) with the aims of comparing relevant clinical outcomes (that is, visual analog scores (VAS), total and sub-Western Ontario and McMaster Universities Osteoarthritis index (WOMAC) scores, Lequesne algofunctional index, joint space width change, and adverse events) between diacerein, glucosamine, and placebo.
METHODS:
Medline and Scopus databases were searched from inception to 29 August 2014, using PubMed and Scopus search engines and included RCTs or quasi-experimental designs comparing clinical outcomes between treatments. Data were extracted from original studies. A network meta-analysis was performed by applying weight regression for continuous outcomes and a mixed-effect Poisson regression for dichotomous outcomes.
RESULTS:
Thirty-one of 505 identified studies were eligible. Compared to placebo, glucosamine showed a significant improvement with unstandardized mean differences (UMD) in total WOMAC, pain WOMAC, function WOMAC, and Lequesne score of -2.49 (95% confidence interval (CI) -4.14, -0.83), -0.75 (95% CI: -1.18, -0.32), -4.78 (95% CI: -5.96, -3.59), and -1.03 (95% CI: -1.34, -0.72), respectively. Diacerein clinically improves visual analog scores, function WOMAC, and stiffness WOMAC with UMD values of -2.23 (95% CI: -2.82, -1.64), -6.64 (95% CI: -10.50, -2.78), and -0.68 (95% CI: -1.20, -0.16) when compared to placebo.
CONCLUSIONS:
The network meta-analysis suggests that diacerein and glucosamine are equally efficacious for symptom relief in knee OA, but that the former has more side effects.
Sci Rep. 2015 Nov 18;5:16827. doi: 10.1038/srep16827.
Effectiveness and safety of Glucosamine, chondroitin, the two in combination, or celecoxib in the treatment of osteoarthritis of the knee.
Zeng C1, Wei J2,3, Li H1, Wang YL1, Xie DX1, Yang T1, Gao SG1, Li YS1, Luo W1, Lei GH1.
Author information
Abstract
This study aimed to investigate the effectiveness and safety of glucosamine, chondroitin, the two in combination, or celecoxib in the treatment of knee osteoarthritis (OA). PubMed, Embase and Cochrane Library were searched through from inception to February 2015. A total of 54 studies covering 16427 patients were included. Glucosamine plus chondroitin, glucosamine alone, and celecoxib were all more effective than placebo in pain relief and function improvement. Specifically, celecoxib is most likely to be the best treatment option, followed by the combination group. All treatment options showed clinically significant improvement from baseline pain, but only glucosamine plus chondroitin showed clinically significant improvement from baseline function. In terms of the structure-modifying effect, both glucosamine alone and chondroitin alone achieved a statistically significant reduction in joint space narrowing. Although no significant difference was observed among the five options with respect to the three major adverse effects (withdrawal due to adverse events, serious adverse events and the number of patients with adverse events), the additional classical meta-analysis showed that celecoxib exhibited a higher rate of gastrointestinal adverse effect comparing with the placebo group. The present study provided evidence for the symptomatic efficacy of glucosamine plus chondroitin in the treatment of knee OA.
BMJ. 2010 Sep 16;341:c4675. doi: 10.1136/bmj.c4675.
Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis.
Wandel S1, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ, Reichenbach S, Trelle S.
Author information
Abstract
OBJECTIVE:
To determine the effect of glucosamine, chondroitin, or the two in combination on joint pain and on radiological progression of disease in osteoarthritis of the hip or knee. Design Network meta-analysis. Direct comparisons within trials were combined with indirect evidence from other trials by using a Bayesian model that allowed the synthesis of multiple time points.
MAIN OUTCOME MEASURE:
Pain intensity. Secondary outcome was change in minimal width of joint space. The minimal clinically important difference between preparations and placebo was prespecified at -0.9 cm on a 10 cm visual analogue scale.
DATA SOURCES:
Electronic databases and conference proceedings from inception to June 2009, expert contact, relevant websites. Eligibility criteria for selecting studies Large scale randomised controlled trials in more than 200 patients with osteoarthritis of the knee or hip that compared glucosamine, chondroitin, or their combination with placebo or head to head. Results 10 trials in 3803 patients were included. On a 10 cm visual analogue scale the overall difference in pain intensity compared with placebo was -0.4 cm (95% credible interval -0.7 to -0.1 cm) for glucosamine, -0.3 cm (-0.7 to 0.0 cm) for chondroitin, and -0.5 cm (-0.9 to 0.0 cm) for the combination. For none of the estimates did the 95% credible intervals cross the boundary of the minimal clinically important difference. Industry independent trials showed smaller effects than commercially funded trials (P=0.02 for interaction). The differences in changes in minimal width of joint space were all minute, with 95% credible intervals overlapping zero. Conclusions Compared with placebo, glucosamine, chondroitin, and their combination do not reduce joint pain or have an impact on narrowing of joint space. Health authorities and health insurers should not cover the costs of these preparations, and new prescriptions to patients who have not received treatment should be discouraged.
Rheumatol Int. 2010 Jan;30(3):357-63. doi: 10.1007/s00296-009-0969-5. Epub 2009 Jun 21.
Effect of glucosamine or chondroitin sulfate on the osteoarthritis progression: a meta-analysis.
Lee YH1, Woo JH, Choi SJ, Ji JD, Song GG.
Author information
Abstract
The aim of this study was to assess the structural efficacies of daily glucosamine sulfate and chondroitin sulfate in patients with knee osteoarthritis (OA). The authors surveyed randomized controlled studies that examined the effects of long-term daily glucosamine sulfate and chondroitin sulfate on joint space narrowing (JSN) in knee OA patients using the Medline and the Cochrane Controlled Trials Register, and by performing manual searches. Meta-analysis was performed using a fixed effect model because no between-study heterogeneity was evident. Six studies involving 1,502 cases were included in this meta-analysis, which consisted of two studies on glucosamine sulfate and four studies on chondroitin sulfate. Glucosamine sulfate did not show a significant effect versus controls on minimum JSN over the first year of treatment (SMD 0.078, 95% CI -0.116 to -0.273, P = 0.429). However, after 3 years of treatment, glucosamine sulfate revealed a small to moderate protective effect on minimum JSN (SMD 0.432, 95% CI 0.235-0.628, P < 0.001). The same was observed for chondroitin sulfate, which had a small but significant protective effect on minimum JSN after 2 years (SMD 0.261, 95% CI 0.131-0.392, P < 0.001). This meta-analysis of available data shows that glucosamine and chondroitin sulfate may delay radiological progression of OA of the knee after daily administration for over 2 or 3 years. Arthritis Care Res (Hoboken). 2014 Dec;66(12):1844-55. doi: 10.1002/acr.22376. Risk of bias and brand explain the observed inconsistency in trials on glucosamine for symptomatic relief of osteoarthritis: a meta-analysis of placebo-controlled trials. Eriksen P1, Bartels EM, Altman RD, Bliddal H, Juhl C, Christensen R. Author information Abstract OBJECTIVE: To determine whether study sponsor, chemical formulation, brand of glucosamine, and/or risk of bias explain observed inconsistencies in trials of glucosamine's efficacy for treating pain in osteoarthritis (OA). METHODS: A systematic review and stratified meta-analysis of randomized placebo-controlled trials was performed, and random-effects models were applied with inconsistency (I(2) ) and heterogeneity (tau(2) ) estimated using Review Manager and SAS, respectively. The major outcome was reduction of pain; the standardized mean difference (SMD [95% confidence interval (95% CI)]) served as effect size. RESULTS: The inclusion criteria yielded 25 trials (3,458 patients). Glucosamine moderately reduced pain (SMD -0.51 [95% CI -0.72, -0.30]), although a high level of between-trial inconsistency was observed (I(2) = 88%). The single most important explanation (i.e., covariate) was brand, reducing heterogeneity by 41% (P = 0.00032). Twelve trials (1,437 patients) using the Rottapharm/Madaus product resulted in significant pain reduction (SMD -1.07 [95% CI -1.47, -0.67]), although a sensitivity analysis of 3 low risk of bias trials using the Rottapharm/Madaus product showed less promising results (SMD -0.27 [95% CI -0.43, -0.12]), which is only a small effect size. Thirteen trials (1,963 patients) using non-Rottapharm/Madaus products consistently failed to show a reduction in pain (SMD -0.11 [95% CI -0.46, 0.24]). The second most important explanation was overall risk of bias (reducing heterogeneity by 32%). CONCLUSION: Most of the observed heterogeneity in glucosamine trials is explained by brand. Trials using the Rottapharm/Madaus glucosamine product had a superior outcome on pain in OA compared to other preparations of glucosamine. Large inconsistency was found, however. Low risk of bias trials, using the Rottapharm/Madaus product, revealed a small effect size.
What is the deal with Neurontin/gabapentin being prescribed for mild/moderate arthritic pain in dogs. Less side effects than NSAIDS but does it work?
My small breed is 5 years old and had an odd gait when I got him, he was a little less than 1 year old, pediatric neuter and had been cooped up in a pen.
He responded well to slow but steady walks (up to 4-5 miles)
Lately he has begun to limp once or twice a week for a day (tends to favor and not put weight on one hind leg) if he walks more than 2 miles.
If I don’t walk him he is better in about 2 days, then it all starts again.
Should I opt for x-rays, additional testing? Or, aqua therapy once a twice a week (if I can afford it) and prn Neurontin?
I had him looked at a while back, hips okay, no anomalies observed.
I was advised if it got worse to go for additional diagnostic testing.
However, He has an excellent appetite and will break into a full trot if he sees a squirrel…….so maybe he is not that uncomfortable?
Update: Had the x-rays done, results unremarkable, exam unremarkable.
So, the vet suspects mild arthritis and recommends glucosamine and fish oil supplements, says I should buy them through his office as a lot of them are not what they say, they are not FDA regulated.
Well, I already give them fish oil, will try a glucosamine supplement I found on Amazon as I really don’t believe in it……. we’ll see
I think swimming helps, if you are lucky enough to be able to afford hydrotherapy or live near a clean lake that allows dogs.
Tried the glucosamine on the recommendation of my vet. Sure enough 2-3 weeks in the possible side effect of GI upset, vomiting began. Pretty sure the glucosamine is the culprit. Hoping symptoms will resolve within a day or two…..
Most supplements suck, except for fish oil.
How exactly do I go about addressing this with my vet? My dog has suffered from arthritis for a while now and some time back she was put on fish oil and glucosamine and chondroitin. I didn’t question it at the time but I’ve noticed no benefit and having read what you’ve had to say about it I feel you put forward a very compelling case.
The problem is when I brought up my concerns about a lack of evidence for glucosamine and my desire to get more effective treatment while she did prescribe gabapentin to try she insisted I keep my dog on the glucosamine and said it would help prevent further degeneration. As someone who isn’t a medical professional I don’t feel like I’m on equal ground to argue with her, and “I read about it on the internet” is rarely considered a compelling argument.
On one hand, of course, you don’t really need to make an argument. You are the client and the owner, and you make all the decisions about care, so you could simply say you don’t see a benefit or any reason to believe there is one and that you don’t intend to continue giving the glucosamine.
If, however, you want to make an effort to convince your vet or defend your decision, there are a number of published scientific papers making this case, several of which are cited in this post, and you could simply provide the references to show that this is not just the opinion of Dr. Google but the conclusion of mainstream scientific experts published in the veterinary literature.
Not to make your life harder, but there is almost no evidence in people that gabapentin is useful for arthritis pain (it is mostly used for neuropathic pain), and there is none in dogs. It is popular now the way tramadol was until recently because it is oral and relatively safe. However, tramadol has since been shown not to work, and until there is some evidence supporting gaba for this use, I think it is unfortunate so many vets are using it so often. The best, proven medication for arthritis are non-steroidal anti-inflammatory drugs (NSAIDs), and there is a huge basis of evidence for the safety and efficacy of these in humans and dogs. Ecept for some very limited situations, these should always be the first choice, so I hope your dog is taking one if possible.
Thank you so much for continuing to reply to this posting, despite the fact that the original post was so long ago. I keep checking back here, in case the evidence has changed. I see it hasn’t.
Hello!
Thanks for piling up this information. In the profession, we have been hearing about the lack of evidence for glucosamine/chondroitin supplements when treating OA in dogs for a long time. I do think that there is an inherent difficulty in coming up with trials (placebo controlled or otherwise) to assess for a beneficial response of said supplement. There is variety of limitations in assessing pain/discomfort, although pain score assessments have been validated, they may prove to be inadequate in OA cases.
Clearly, when a pet has OA, as evidenced by radiographs, the use of NSAIDs is the treatment of choice (along with weight loss, and low impact exercise, etc).
The question to me is… can glucosamine supplements be helpful in dogs “prone” to OA, or dogs that are “a little stiff”, have some muscle atrophy, but no evidence of OA on radiographs…? Can they be helpful in mitigating progression of joint disease? They may not be indicative as pain control treatments, but they may have beneficial properties regardless.
I have heard plenty of times owners giving positive reviews of this or that glucosamine supplement (whether recommended by a vet or OTC), saying it has made a big difference in their dog’s mobility. I am well aware of placebo effect and the limitations of anecdotal evidence, but if perceptually the owner is telling me it has made a difference, who am I to question that!? I think ultimately these supplements are on the “can’t hurt, may help” category. As an aside note, the scientific community has nothing to say about the implications of the placebo effect (how can we explain it or what are its ramifications!?).
Regarding the use of NSAIDs, despite being overall safe, we need to keep in mind their potential harmful side effects, relating to the effects on the mucosal barrier (there are countless of episodes of mucosal ulceration in people yearly) as well as their potential harmful effects on the kidneys. Hopefully, the newer more selective COX inhibitors end up being all they claim to be.
Cheers!
You are the patient’s advocate, and it is a critical part of our job as vets to push for what is best for thee patient even if the owner has a different perception of this! Owners have great love for their pets and ultimate decision-making power, but they also have their own perspective and agendas that often don’t align with what the animal needs. And they certainly do not have our training or experience in interpreting signs and symptoms. How many dogs and cats have I seen looking emaciated and having lost 10%, 20%, even 30% of their body weight without the owner noticing?! Owners are not infallible interpreters of their pets’ needs, and we shouldn’t hesitate to let them know when their perceptions may be inaccurate, whether due to placebo effects or other causes.
I’m not sure what you mean. The placebo effect is the subject of tons of active scientific research, and we have a LOT to say about it and how it misleads us in research and in clinical medicine. As you can see on this blog, I wrote a book with large sections devoted specifically to how placebo effects fool us into believing in the benefits of implausible or ineffective therapies.
There is. no evidence for this. Many studies in humans and lab animals have looked at progression of disease as well as pain and found no reliable evidence that glucosamine affects the development of arthritis any more than the symptoms. It is nearly impossible to prove beyond any doubt that a preventative therapy isn’t working with a chronic, gradually progressive, and largely unpredictable disease like OA, but the absence of evidence after decades of testing sure makes me wonder if the money we waste of this supplement, and the tendency for owners to use it before or instead of other therapies, isn’t doing more harm than any possible good it might be doing.
I’ve moved into the “can’t hurt, probably don’t help” category, both because of the many years of research evidence and two decades of seeing patients taking these supplements. The harm they do isn’t direct, but again if using them delays or replaces truly effective treatment, there is definitely harm to patients.
Appreciate the response.
“Owners are not infallible interpreters of their pets’ needs, and we shouldn’t hesitate to let them know when their perceptions may be inaccurate, whether due to placebo effects or other causes.” – Point taken, as this is true in some cases. That being said, I see no harm done if the owner wants to have the dog on a “joint supplement”, as long as they understand this is not a treatment for pain. The only harm is potentially in their wallet. I think everybody agrees glucosamine is not a substitute to analgesics. After all, treating OA is a multimodal approach. There are cases in which an NSAID trial shields minimal improvement perceptually, so in that case, should we insist the owner in administering a medication because the research indicates it should help, despite the lack of results they may witness? Ultimately, NSAIDs treat symptoms, but won’t remodel the arthritic joint. Now when we introduce physical therapy (ROM exercises, low impact activity, etc), we may see more long-lasting beneficial effects.
To briefly touch on the placebo effect, I know this is a recognized phenomenon. My comment was regarding how reductionist science grapples with an explanation for a psychosomatic effect. Sometimes the illness may have been bound to get better without intervention, thus solving the mystery of placebo. But in other cases, it may provide more insight into the mind/body connection. I admit to have not read your book, so I may need to check that out at some point. The conversation on the placebo effect is probably beyond the scope of this discussion on glucosamine.
Cheers!
Yes the placebo is a complex and fascinating topic! Some do argue for a mind-over-matter interpretation, suggesting that real healing occurs due to the effects of belief/expectancy and other mental phenomena. I don’t think this is a well-supported interpretation of the evidence. I think the key is that placebos influence our perception of disease and have some effects on autonomic responsive connected with our perceptions (heart rate, blood pressure, etc.). These effects may reduce subjective symptoms (pain, nausea, etc), but they do not fundamentally alter the course of organic disease.
The argument in humans then becomes, do the potential benefits in how patients experience their illness outweigh the potential harms of placebos (the probable necessity for deception, the tendency to trust anecdotal experience excessively to the point where we accept the efficacy of clearly ineffective therapies, the potential to delay or replace effective Tx, etc.). In veterinary patients, there is not much to support the idea that even these benefits pertain to our patients. Apart form some autonomic effects of human contact, pets don’t experience placebo effects based on belief or expectation, so the perception of improvement is almost entirely due to owner beliefs, regression to the mean, Hawthorn effect, and other variables that don’t directly improve the underlying condition. I think there is a powerful ethical argument against placebos in pets because it is too easy for owners and vets to perceive an improvement that isn’t there and then allow unnecessary suffering. I have seen animals in terrible pain who’s owners were convinced the homeopathy or acupuncture or whatever was working and could not be convinced otherwise.
Here is an article talking about the issue that doesn’t require you to buy the book. 😉
Here in mexico virbac sells a product “chondroflex”
Is there any diference with another suplements with glucosamine+ chondrotin?
Nope
The CVMA has Cartrophen Vet (injections of Pentosan polysulphate) recommended. The web site reports it is not an analgesic, but instead supports joint fluid production, which may lessen the signs of OA in dogs.
I wonder if you have ever looked at Cartrophen?
Seems the only options we can find for our boy with already elevated kidney enzymes is Cartrophen, what my vet calls Metacam pulsing or stem cell injections. Thank you for your time.
Update:
I found the new NSAID called Galliprant on the FDA web site and sent it to my vet.
She is going to order it for us to try and said, unlike other NSAIDs, it should have very little effect on his kidneys & liver. Still some side-effects to monitor, however.
https://www.fda.gov/animal-veterinary/animal-health-literacy/galliprant-nonsteroidal-anti-inflammatory-drug-nsaid-dogs-osteoarthritis
Thank you again for your time and your articles.
What if I supplement my healthy dog’s food with Glucosamine either through a soft chew supplement or a can of Royal Canin Joint Care? Just for general benefit in a healthy dog, not to treat an existing illness.
There’s no evidence glucosamine has any “general benefit,” and it would be hard to imagine why it would.