I recently reported on the results of a clinical trial conducted at North Carolin State University on the purported “anti-aging” Leap Years. The study provided no convincing evidence of a beneficial effect, and despite a single statistically significant finding at one time point, the data looked about as clearly negative as a study measuring multiple outcomes like this can.
Despite this, the company and its most prominent figure, Dr. David Sinclair, promoted it heavily as a major advance in canine geroscience. The pushback for these excessive and unsupported claims was surprisingly strong, and Dr. Sinclair had to resign the presidency of a major aging research organization he belongs to. That said. the product is still for sale and the company still makes many unproven claims for it, including some specifically based on this paper.
The original manuscript was released as a preprint, which is not peer reviewed, so when such studies are eventually published (if they are), sometimes the manuscript can change based on reviewers comments. The published version of this paper has now appeared, and there are pretty minimal changes.
As an example, here are the titles and abstracts from the two versions, with the differences bolded-
Preprint-
Randomized, Controlled Clinical Trial Demonstrates Improved Cognitive Function in Senior Dogs Supplemented with a Senolytic and NAD+ Precursor Combination
Peer-reviewed-
A randomized, controlled clinical trial demonstrates improved owner-assessed cognitive function in senior dogs receiving a senolytic and NAD+ precursor combination
Preprint-
There was a significant difference in CCDR score across treatment groups from baseline to the primary endpoint (p=0.02) with the largest decrease in the full dose group. There were no significant differences between groups in changes in measured activity. However, the proportion of dogs that improved in frailty and owner-reported activity levels and happiness was higher in the full dose group than other groups. Adverse events occurred equally across groups. All groups showed improvement in cognition, frailty, and activity suggesting placebo effect and benefits of trial participation. We conclude that LY-D6/2 significantly improves owner-assessed cognitive function and may have broader effects on frailty, activity and happiness as reported by owners.
Peer-reviewed-
There was a significant difference in CCDR score across treatment groups from baseline to the primary endpoint (p = 0.02) with the largest decrease in the full dose group. No difference was detected between groups using in house cognitive testing. There were no significant differences between groups in changes in measured activity. The proportion of dogs that improved in frailty and owner-reported activity levels and happiness was higher in the full dose group than other groups, however this difference was not significant. Adverse events occurred equally across groups. All groups showed improvement in cognition, frailty, and activity suggesting placebo effect and benefits of trial participation. We conclude that LY-D6/2 improves owner-assessed cognitive function over a 3-month period and may have broader, but more subtle effects on frailty, activity and happiness as reported by owners.
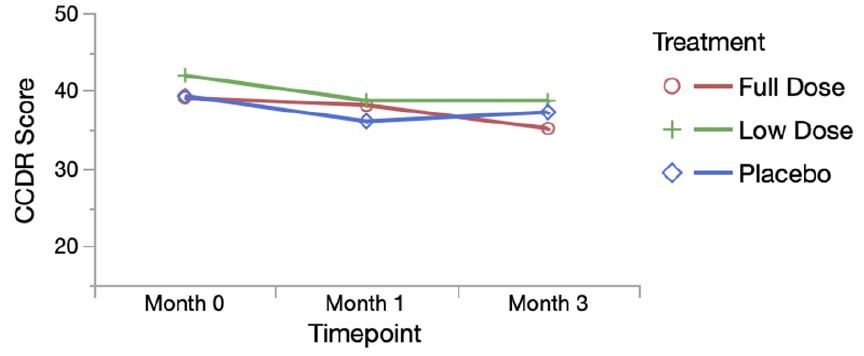
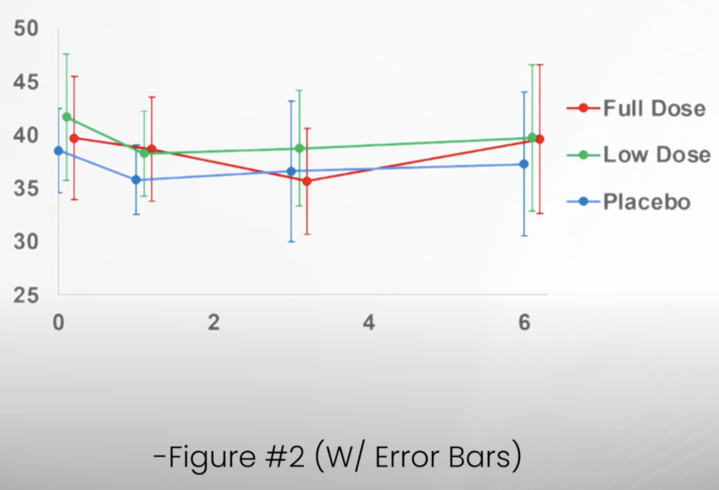
The intention of these small changes seems to be to walk back the level of confidence given for some of the findings and their significance. Similar changes appear in the discussion and elsewhere in the paper, but they don’t fundamentally change the reporting of results. I was a bit surprised that the reviewers did not recommend error bars be added to figure or that these be extended to include the 6-month evaluation. Both of these improvements to the figures emphasize the lack of a consistent and coherent pattern of response that would support meaningful benefits to the subjects.
Dr. Matt Kaeberline, an aging biology expert and strong critic of Dr. Sinclair, has prepared an amended figure based on the data the authors made available. The comparison between that figure and the one in the ;published article emphasizes how easy it is to present the data in one way and see the appearance if a pattern which is much less evident when the same data are presented in a different way.
Figure from paper-
Fire with error bars and 6-month data-
Bottom Line
The published paper is very similar to the preprint, which is not surprising since the original study was well-designed and conducted. The best case that can be made is that the supplement might have som effect on cognitive function in older dogs, but the existing evidence is underwhelming and more negative than positive overall. This might be enough to support further testing, but it certainly is not strong or sufficient to justify ongoing use, much less exuberant marketing of the product as an “anti-aging” treatment.










My dog developed pancreatitis which resulted in renal failure and death after taking this supplement for 8 months. Other dog owners on Reddit had the same unfortunate experience.
What happened is sad but that doesn’t prove Leap Years was the cause. Pancreatitis can come on quickly for no apparent reason.
Very true. Of course, without adequate safety testing, it is difficult to know whether or not the product has any effect on the risk of this or other health problems. The data are so far limited, and the negative anecdotes have to be given the same weight (minimal) as the positive anecdotes. Their main role is to suggest effects we should then test for in a controlled way.