One of the longest running subjects for posts here has been the Chinese herbal product Yunnan Baiyao (YB). Purported to help stop or prevent bleeding, this stuff seems to be pretty commonly used in veterinary medicine, mostly for dogs with an aggressive form of cancer prone to bleeding, hemangiosarcoma (HSA). Despite this popularity, and the perception of “well, it can’t hurt,” there is very limited research evidence on YB, and what exists is not encouraging.
My first review was in 2010, and my most recent in 2017 (2010, 2016, Feb 2017, June 2017). In the latest, my conclusions were largely the same as in the first:
The TCM rationale for using Yunnan Baiyao is part of an unscientific, quasi-religious belief system and cannot be accepted as a sufficient basis for using an otherwise unproven remedy on patients, especially when the ingredients in that remedy are not fully disclosed or regulated for quality, consistency, and safety. The more plausible scientific hypotheses for how Yunnan Baiyao might work remain unproven.
The clinical research evidence is mostly negative, and even positive studies have not shown any significant effects on clinically meaningful objective outcomes. No clear evidence of harm has yet been found, though the limited nature of the evidence does not ensure that the product is truly safe.
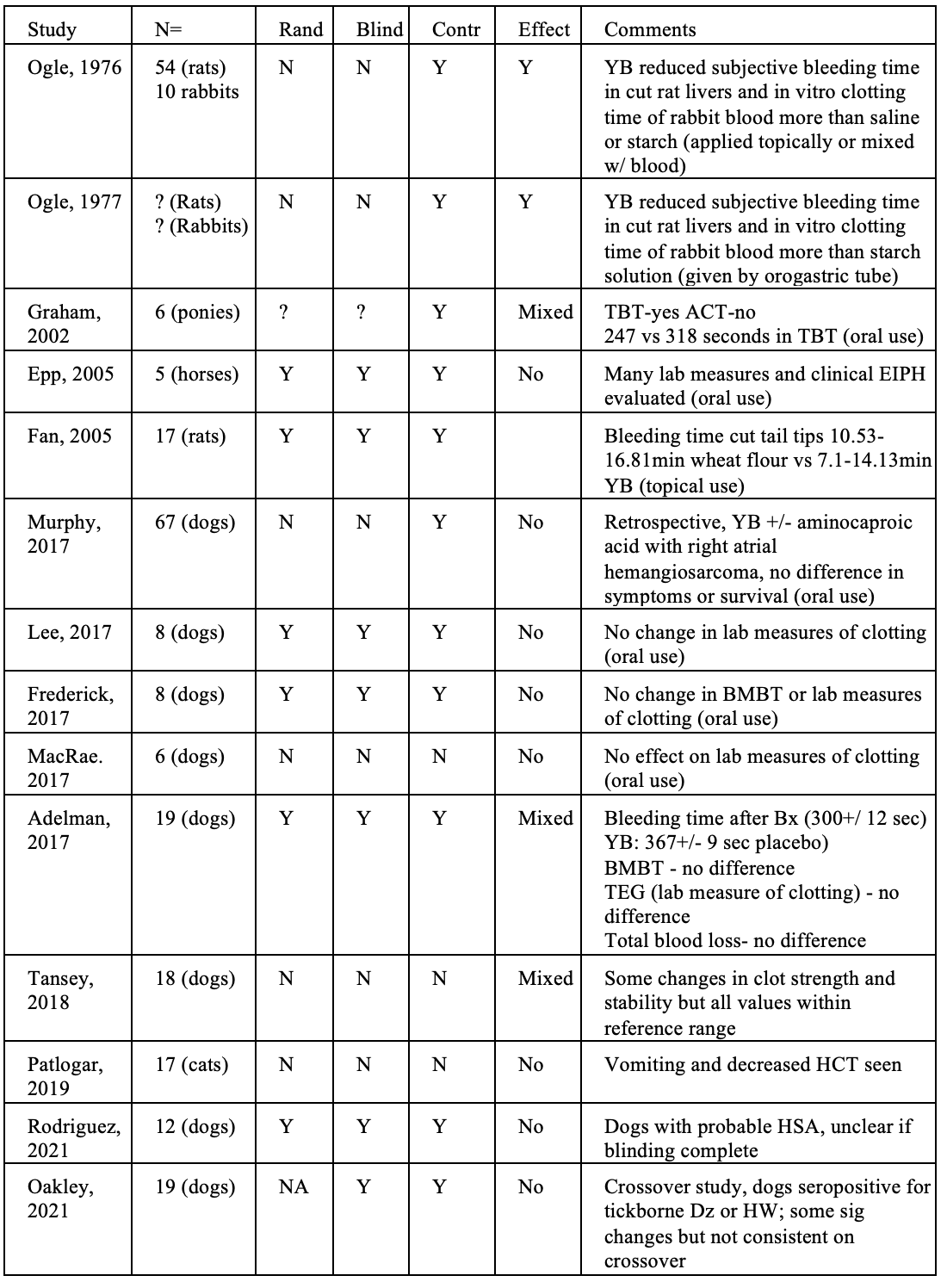
I also created a table listing all of the animal studies I had been able to find, with the following summary- “Of these ten studies, 5 found no effect at all, and 2 others showed mixed results, with possible effects in some measures evaluated in the studies but not in others. Of the three fully positive studies, two did not report any of the major methods for controlling for possible bias and other sources of error.”
New Evidence
Given it has been seven years since I last addressed the topic, I took a look for new animal studies done since then, and I have found several, which I will review here.
- Tansey C, Wiebe ML, Hybki GC, Patlogar JE, Murphy LA, Bianco D, Nakamura RK. A prospective evaluation of oral Yunnan Baiyao therapy on thromboleastographic parameters in apparently healthy dogs. J Vet Emerg Crit Care (San Antonio). 2018;28(3):221-225.
This study evaluated laboratory measures of blood clotting in 18 apparently healthy dogs of various breeds (mostly large dogs, with an average size of over 60lbs). All dogs received 250mg of the product twice a day (5.8–13.4 mg/kg) for 1 week, with no blinding or control group. A large number of in vitro measures of clotting were evaluated (see the table) with a few statistically significant changes from baseline.
- “TEG changes consistent with increased clot strength and reduced fibrinolytic activity. may suggests that YB produced an increased in clot strength, although these parameters remained within the reference interval.”
- “YB also apparently reduced fibrinolytic activity as evident by the significantly reduced values for LY30 and LY60 noted at 1 week recheck; changes in these parameters remained within the reference range as far as we could determine.”
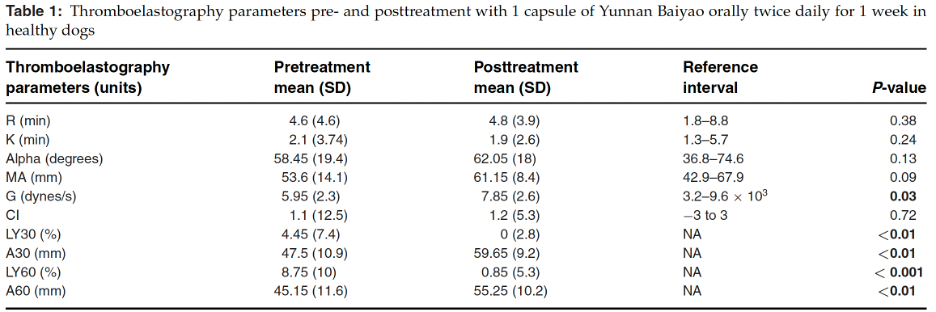
No significant changes were seen in any other measures, including those related to platelet function, which has previously been claimed to be the suspected mechanism by which YB might affect clotting. The authors pointed out that these results were inconsistent with a previous study in beagles (Lee, 2017) and suggested the differences could be due to different methods for testing blood clotting or differences in the YB products used since there is very little consistency and no reliable quality control guidelines for the production of Chinese herbal medicines.
No apparent adverse effects were noticed.
2. Patlogar JE, Tansey C, Wiebe M, Hybki GC, Trostel T, Murphy LA, Nakamura RK. A prospective evaluation of oral Yunnan Baiyao therapy on thromboelastographic parameters in apparently healthy cats. J Vet Emerg Crit Care (San Antonio). 2019;29(6):611-615.
The same group also conducted a very similar study in cats. Seventeen healthy cats were enrolled, and most received the same 250mg dose of YB twice a day for a week, a much higher dose relative to body weight than the dogs were given (31.71–90.91 mg/kg).
No significant changes in any of the numerous measures of blood clotting were seen in these cats. However, the cats receiving YB did have a significant decrease in their red blood cell count (HCT) and a close-to-significant decrease in weight. Once cat dropped out due to severe vomiting after receiving YB, and two others has some vomiting but were able to complete the study. Though the HCT remained in the reference range, these changes suggest that YB at this dose could have detrimental effects on cats especially if given for a longer time.
3. Rodriguez A, Shiau D, Levinson K, et al. Preoperative oral administration of Yunnan Baiyao and its effect on coagulation parameters in tick-borne disease and/or heartworm seropositive dogs: A pilot study. Am J Trad Chin Vet Med 2021; 16(2):23-30.
In a rather odd study, published in a journal dedicated to so-called Traditional Chinese Veterinary Medicine (TCVM), 12 dogs scheduled for neutering and also known to be positive for antibodies showing exposure to several tickborne diseases or canine heartworm disease (though actual infection was not determined) were given YB. The rationale was that these patients might be more likely to bleed during surgery, though there is limited evidence showing this.
The dogs were randomly divided into equal groups to receive either YB or a placebo, and some individuals were blinded to treatment, though it is not clear if this included those running the tests, doing the surgery and estimating blood loss, or analyzing results. The dogs were given approximately 100mg/kg every 12 hours for three doses.
Multiple lab tests assessing clotting were run, and a visual estimate was made of blood loss during surgery. No statistically significant differences were seen in any measure except for a decrease in prothrombin time (an indicator of increased clotting ability) in the placebo group. The authors interpret the non-significant differences seen as suggesting, on the whole, a pro-clotting effect, but in a small study with no statistically significant results, this is only a hypothesis, not a valid conclusion.
4. Oakley C. Bruyette D. Chretin J. Hazzah T. Bergman P. Thromboelastography to evaluate coagulation changes in dogs on Yunnan Baiyao with hemangiosarcoma: a pilot study. J Amer Hol Vet Med Assoc. 2021;64:49.
I have not been able to obtain a full-text copy of this article, published in the rather notoriously unscientific journal of the American Holistic Veterinary medical Association, so I cannot properly evaluate the methods. Based on the abstract alone, this was a double-blinded crossover study of 19 dogs with confirmed (14) or suspected (5) HSA. The dogs were given the chemotherapy agent doxorubicin and then 60mg/kg per day of a YB product or a placebo. At 21 days, the dogs were switched from one treatment (YB or placebo) to the other. Multiple blood tests were run at baseline and days 7, 21, 28, and 42 of treatment.
The handful of statistically significant findings reported in the abstract are difficult to interpret without additional information. Some differences between groups were seen in some measures on some days that might suggest a pro-clotting effect, but whether there was an overall pattern suggesting a real effect, and whether this was clinically relevant to the dogs, is unclear.
The final statements of the abstract suggest that a real effect is unlikely since the changes seen were not consistent when the dogs were switched from one treatment to the other:
Although patients on YB in the first leg of the study were more hypercoagulable and patients on YB in the second leg of the study had a higher red cell count than those on placebo, these findings were not repeatable via crossover. TEG did not detect consistent coagulation changes as it relates to treatment with YB.
Based on assessment of these new studies, I have updated by tabulation of animal study results:
What Does It All Mean?
This brings the total of studies I have seen to 14. Of these, a majority (8) are clearly negative, and the others mostly show mixed results. Many have significant methodological limitations that make the real-world relevance of their findings questionable.
There is also inconsistency in the results between studies. Positive findings in one are often contradicted by negative results in another. Despite decades of animal research, no consistent pattern has emerged showing even a clear and verifiable mechanism of action, much less any meaningful clinical benefits in real patients.
While few studies have reported significant negative side-effects (except for the relatively high-dose study in cats), the available literature does not convincingly address significant concerns about safety. The composition of YB in general, much less individual products or different batches of the same product, is not clearly established, and there is no meaningful quality control to ensure consistency or the absence of harmful contaminates. There is a large literature showing that Traditional Chinese Medicines often contain dangerous ingredients or contaminates (e.g. 1, 2), which cause significant harm to patients (e.g. 3, 4), and there is no assurance that YB is free of these. The blithe assumption of safety for supposedly “natural” herbal products is unjustified, and the idea that no harm can be done in patients with advanced neoplasia has also been shown to be wrong (e.g. 5).
The literature evaluating YB has also never provided a cogent answer to the question of how promoting clotting, if the product even does this, could occur without a meaningful risk of thromboembolic events (undesirable clots such as those seen in strokes in humans and in feline aortic thromboembolism in cats with heart disease). If YB increases clot formation enough to meaningfully reduce bleeding, even the serious life-threatening hemorrhage seen in dogs with HSA, how does it do this without causes unwanted clots? The idea that you can have benefits without risks is common in alternative medicine, but science-based medicine knows better, and so should we as vets.
When a product has been studied for over 40 years without showing even a clear and consistent effect, much less a meaningful clinical benefit, there is little justification for using it in our patients. Further and better research may be justified, though on pretty tenuous grounds at this point, but at this point we are rolling the dice blindly every time we give YB to a patient. If we are not clear about this uncertainty with clients, then we are misleading them and denying them the basic right to fully informed consent. The psychological drive to grasp at straws when we are desperate is powerful and understandable, but it is not a rational or appropriate basis for the practice of medicine.
References
Ogle CW, Soter D, Cho CH (1977) The haemostatic effects of orally administered yunnan bai yao in rats and rabbits. Comparative Medicine East and West 5:2, 155-160
Ogle CW, Dai S, Ma JC. The haemostatic effects of the Chinese herbal drug Yunnan bai yao: A pilot study. Am J Chin Med (Gard City N Y) 1976;4:147–152.
Graham L, Farnsworth K, Cary J (2002) The effect of yunnan baiyao on the template bleeding times and activated clotting times in healthy ponies under halothane anesthesia.Journal of Veterinary Emergency and Critical Care 12:4; 279; 2002 (abstract only)
Epp TS, McDonough P, Padilla DJ, et al. The effect of herbal supplementation on the severity of exercise-induced pulmonary haemorrhage. Equine and Comparative Exercise Physiology 2005;2:17-25.
Fan C, Song J, White CM. A comparison of the hemostatic effects of notoginseng and yun nan baiyao to placebo control. J Herb Pharmacother 2005;5:1–5
Murphy LA, Panek CM, Bianco D, Nakamura RK. Use of Yunnan Baiyao and epsilon aminocaproic acid in dogs with right atrial masses and pericardial effusion. J Vet Emerg Crit Care (San Antonio). 2016 Sep 26. doi: 10.1111/vec.12529. [Epub ahead of print]
Frederick J, Boysen S, Wagg C, Chalhoub S. The effects of oral administration of Yunnan Baiyao on blood coagulation in beagle dogs as measured by kaolin-activated thromboelastography and buccal mucosal bleeding times. Can J Vet Res. 2017 Jan;81(1):41-45.
Lee A. Boysen SR. Sanderson J. et al. Effects of Yunnan Baiyao on blood coagulation parameters in beagles measured using kaolin activated thromboelastography and more traditional methods. International Journal of Veterinary Science and Medicine. 2017;5(1):53–56.
MacRae R. Carr A. The Effect of Yunnan Baiyao on the Kinetics of Hemostasis in Healthy Dogs. ACVIM Forum, National Harbor, MD, 2017.
Adelman L. Olin S. Egger CM. Stokes JE. Effect of Oral Yunnan Baiyao on Periprocedural Hemorrhage and Coagulation in Dogs Undergoing Nasal Biopsy. ACVIM Forum, National Harbor, MD, 2017.
Tansey C, Wiebe ML, Hybki GC, Patlogar JE, Murphy LA, Bianco D, Nakamura RK. A prospective evaluation of oral Yunnan Baiyao therapy on thromboleastographic parameters in apparently healthy dogs. J Vet Emerg Crit Care (San Antonio). 2018;28(3):221-225.
Patlogar JE, Tansey C, Wiebe M, Hybki GC, Trostel T, Murphy LA, Nakamura RK. A prospective evaluation of oral Yunnan Baiyao therapy on thromboelastographic parameters in apparently healthy cats. J Vet Emerg Crit Care (San Antonio). 2019;29(6):611-615.
Rodriguez A, Shiau D, Levinson K, et al. Preoperative oral administration of Yunnan Baiyao and its effect on coagulation parameters in tick-borne disease and/or heartworm seropositive dogs: A pilot study. Am J Trad Chin Vet Med 2021; 16(2):23-30.
Oakley C. Bruyette D. Chretin J. Hazzah T. Bergman P. Thromboelastography to evaluate coagulation changes in dogs on Yunnan Baiyao with hemangiosarcoma: a pilot study. J Amer Holistic Vet Med Assoc. 2021;64:49.


Pingback: WSAVA Adds Chinese Medicine Pseudoscience to Continuing Education Conference Held in China |
I don’t know if anyone will ever read this. I don’t doubt that the studies above were done. All I know is that my dog was about to be put down over 2 weeks ago with a bleeding tunor. She couldn’t function and the vets told us that she probably wouldn’t last another night. We found Yunnan Biyao from a local Chinese storeowner based on advice of my sister and brother in law (both MDs) who had used it on their Golden Retriever who was suffering from the same condition only a few months ago. My sister’s dog recovered dramatically for around a month after starting this herbal treatment before succumbing to a spread of tumors to her head where it was necessary to put her down. My dog (an 11 year old, 65 lb rescued lab mix) has recovered to the point that she is eating again and is clearly no longer bleeding out. We are getting to spend quality time with her and will likely get to have her for one more Christmas. I am basically certain that this herbal medicine is the reason.
It’s great that your dog has done well. I understand why that experience is so compelling for you, but unfortunately it doesn’t mean the product works, for your dog or anyone else’s. If figuring out which therapies worked and which didn’t were that easy, we wouldn’t need science at all. People have been using this sort of experience to support medical treatments for all of human history, from bloodletting to herbal medicine to ritual sacrifice. And yet we never made any meaningful progress fighting disease or extending lifespan until we stopped relying on stories and started using the scientific method. It’s not perfect, but it’s a lot better.
I know it probably won’t change anything for you, but for anyone willing to consider that things aren’t always what they seem, here are some articles explaining why anecdotes like this can’t be trusted. (and also a cartoon, which is a lot shorter than my posts!)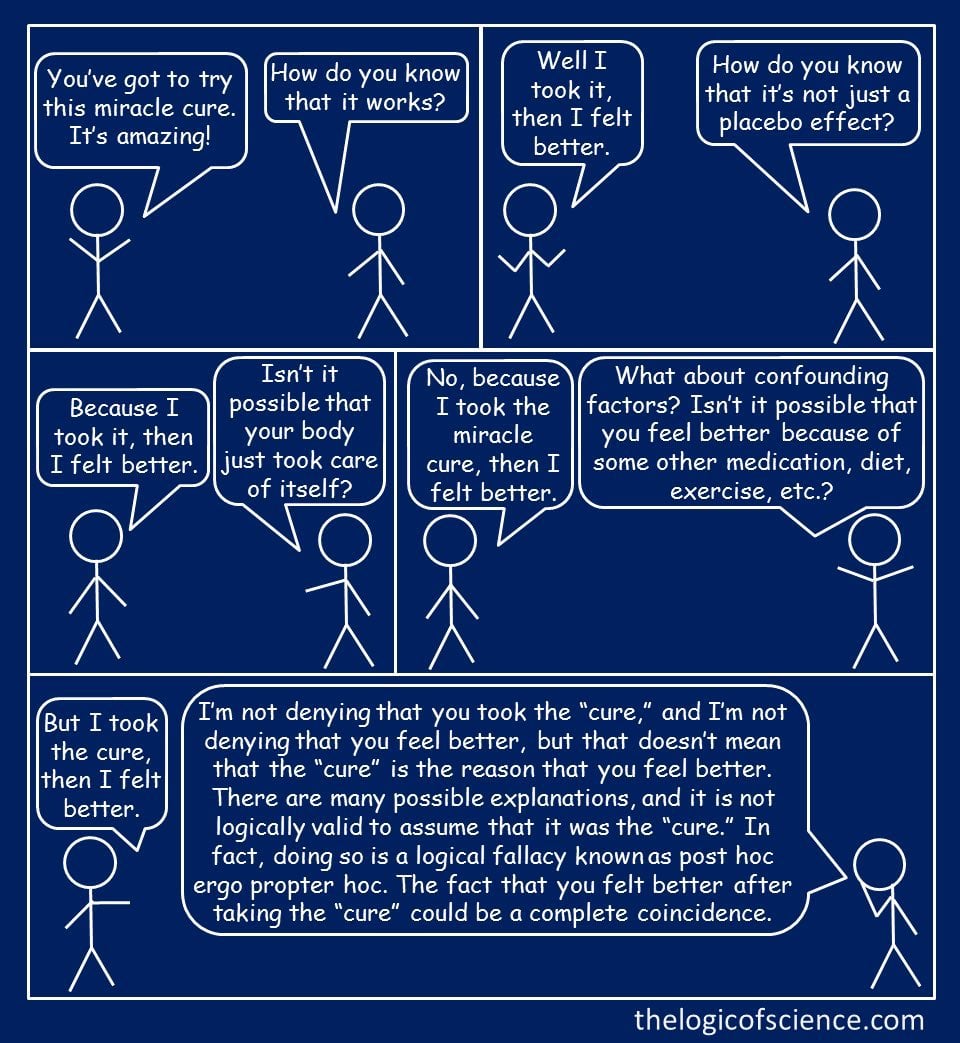
I have a dilemma. Our wonderful 16.5 year old cat Ninja was not eating his normal food, though he was fine with treats and such. We took him in to be checked. The vet referred him to an emergency clinic. He had lost 2 lbs since his August checkup. They did an ultrasound and found multiple liver nodules likely indicative of some sort of cancer as well as blood in the abdomen. His blood work showed anemia but all other markers were fine. They are guessing HSA. We were sent home with prednisone and an appetite enhancer. The covering vet at our primary practice suggested Yunnan Baiyao. We used it for a few days then stopped. Ninja had not been doing well after the covering vet visit but has bounced back. We are getting good appetite and lots of cuddles and jumping up on our bed. We are working with Hospice on maximum quality of life. We understand that the prognosis is dire. We understand that the evidence on YB is really sketchy/anecdotal. But is it worth a try?
I think it is unlikely to help and unlikely to do much harm. I will warn you, though, that “guessing HSA” based on ultrasound can be unreliable (e.g. this paper), so I would keep that in mind as you make any decisions.
Good luck!
I actually just included this topic in a recent mini-talk at our monthly doctors meeting at my hospital. I suggested that we start stocking oral TXA (we have injectable aminocaproic acid for in-hospital use) as an evidence-based alternative to yunnan baiyao. If folks want to continue to use magic dirt, that’s their business, not my place to tell others how to practice. But as a leader in specialty healthcare I think it’s our responsibility to promote medical care that has quality and safety controls, and support in the literature that it actually does what it claims. Btw, hi Dr. McKenzie! Long time! Ten years and I’m no longer a student, now an ER doc pursuing a residency in ECC!
Hi! Nice to hear from you, and nice to hear you are pushing for evidence-based care. Well done!
With respect to HSA, there is a Facebook group with thousands that have been told there was nothing that could be done for their dogs and we have weeks or at best a few months left. Most of us have made it well past that timeframe, I’m going on 8 months with my dog since I was told euthanasia was the best option. Is it just a coincidence that so many of our dogs are living that long without the help of these herbs? Either way it’s a major ball drop on the veterinarian community to suggest euthanasia when they may live many months to years longer. My vet was wrong and so were many others. Our beloved pets are sick but there is hope. Out of pure desperation we are doing our own studies on these herbs, everything from the dosing, what herbs to mix them with to how to make it through a bleed, my dog has had two so far. There is a distrust between us and the veterinarians. We turn to each other because there is no other help. There is so much compassion, sympathy and support, I’ve never seen anything like it. My dog is living his best dog life in this time he has left, I don’t know what happened to give him more time but I’m so glad I didn’t take the advice of my vet. I wish we could come together and figure this out.
Yes, the problem here is mistaking average survival times in a population for predictions that apply to individual pets. The average length of time that dogs with bleeding HSA in the spleen live without any treatment other than surgery is 2-4 months. Does that mean your dog will only live that long if it gets this disease? No one has a clue! The average inlcudes dogs who die before or during surgery (a lot less than 2 months) and dogs that live a year or more.
Unfortunately, it is very hard for vets and dog owners to understand this kind of nuance. I try not to give specific numbers because clients fixate on them and anchor their expectations around them. Figures like these an set a reasonable range of expectations, but they can’t predict the future.
The same is true for repsonse to treatment. On average, dgs liv abou twice as long if given chemotherapy after splenectomy, for example. But some do better than this and some do woirse, and we don’t know how to predit who is who. We don’t have any reason to think YB etends survival at all based on research, but some dogs who take will live more than the average and some less. We tend to hear the stories of the ones who did better than expecte,d and no one talks about their experience when their dog dies earlier than expeted, so anecdotes create a false impression that such treatments are doing something.
The decision of how long is long enough is, of course, yours. It is appropriate for a vet to tell you that in general dogs don’t live more than a few months, but they can’t predict your dog’s future or make that decision for you.