Here is an article from Veterinary Practice News responding to the ridiculous marketing ploy by Petco declaring a ban on “artificial” food ingredients.
Since the late 1980s, individuals and organizations have been trying to warn the public about a deadly chemical known as DHMO. Though widely used in the home and in commercial settings, including the healthcare industry, this substance has been shown to cause severe lung damage and even death if inhaled in small quantities. Hundreds of thousands of people die annually from this cause.1
DHMO can also cause electrolyte disturbances and potentially fatal neurologic symptoms when taken orally, and it can cause severe burns and even explosions when heated.2A number of surveys have found high levels of support for banning DHMO, and elected officials in several countries have explored taking such action, but DHMO remains ubiquitous.3,4
Given the obvious dangers of this chemical, why do public health agencies not take action to restrict it? It is possible that funding and political influence from industry impedes regulatory action. However, it is more likely that governments have chosen not to ban DHMO because it is essential for life. Most people are surprised to learn this until they recognize the non-technical name for this chemical—water.
The campaign against DHMO (dihydrogen monoxide) has been used as a humorous illustration of the problem of chemophobia or chemonoia. These terms refer to the potent and widespread fear of anything labeled a “chemical.”5–7Nonscientists often assume that chemicals are inherently dangerous, even though the word properly refers to nearly every substance we encounter in daily life, from the deadliest poison to the basic necessities of life and even the materials that makes up our own bodies.
A concept integral to chemophobia is the Appeal to Nature Fallacy, the misconception that substances which occur naturally are inherently healthy and safe while those produced by humans, even if chemically identical to natural substances, are dangerous. Of course, it is easy to find examples that belie this notion. Nothing could be more natural than the E. colior Salmonella. Radioactive uranium, asbestos, and cyanide are completely natural.
In contrast, the vaccines which have eliminated smallpox and polio are undeniably artificial. Antibiotics, synthetic vitamin supplements, blood transfusions, organ transplants, prosthetic limbs, insulin for diabetics, and even such simple and unheralded public health technologies as indoor plumbing and toilet paper have saved lives and reduced suffering for millions. Yet these are not “natural” in the usual sense of the word.
Unfortunately, chemophobia and the Appeal to Nature Fallacy are widespread, and they often motivate pet care decisions. Some organizations and individuals take advantage of this by offering “natural” products or therapies and warning of the dangers of “chemicals” and anything “artificial.” A recent high-profile example of this exploitation of chemophobia is the announcement by Petco that the company “will not sell food and treats containing artificial colors, flavors and preservatives for dogs and cats.”8
The company’s public relations materials call this decision “a major step forward for pets” on “a momentous day.”8Petco effectively declares itself the arbiter of what constitutes healthy nutrition, even going so far as offering to “help pet parents affected by such a change to safely transition to a new food or brand that we believe is healthier for their pet” if customers are no longer able to buy a food they have been using.8Even though regulatory agencies and experts around the world have judged the additives on Petco’s list to be safe, the company has decided it knows better.
The dramatic rhetoric in the Petco press materials may serve the purpose of creating a positive and profitable image for the company, but it obscures the danger of a marketing strategy that caters to unscientific reasoning and mostly unfounded fears. There is little in the way of scientific evidence, or even logical consistency, behind the Petco blacklist.9
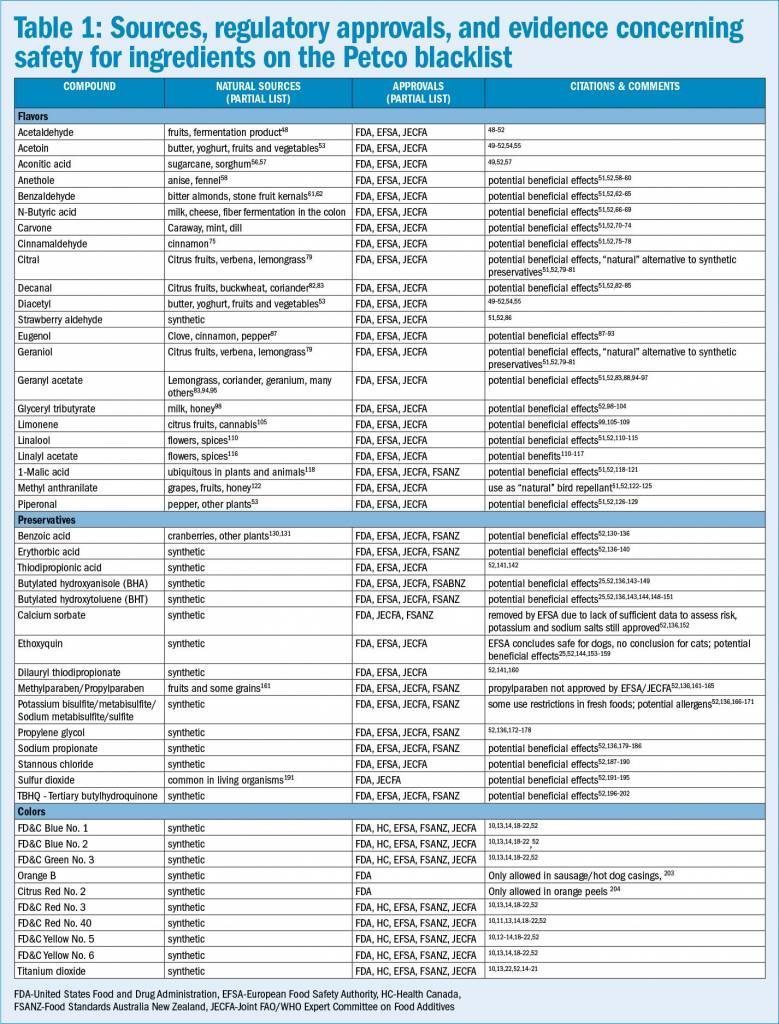
For example, many of the “artificial” flavors and preservatives on the list occur naturally (see Table 1). Of course, the fact that these chemicals occur in nature doesn’t make them safe, just as flavors and preservatives are not necessarily unsafe if produced synthetically. However, the fact that Petco is banning naturally occurring substances for being “artificial” exposes the inconsistent logic behind this blacklist.
The health risks of most substances are related to the dose and route of exposure. And the risk of any substance should always be considered in relation to its benefits. Water is unsafe to drink only in very large quantities, but it is unsafe to breathe in even small amounts. It is also essential for life, taken at appropriate doses and by the appropriate route. The same logic, informed by scientific evidence concerning risks and benefits, should be applied to food additives, but Petco does not use this approach.
Some of the substances on the list have no clear health implications. The color additives, for example, are almost certainly safe, but they serve no nutritional or health purpose.10,11,20–25,12–19These chemicals are added to pet food to appeal to the emotions and aesthetics of pet owners. While they serve no health-related purpose, banning these compounds is itself a way of appealing to the emotions of owners and their irrational fears, and there is no sound reason to believe this will benefit the health of pets.
For other items on the list, the impact of discouraging their use is less clear. Flavorings, for example, make nutritious and affordable commercial foods more palatable. Removing them may make it harder to provider appropriate nutrition to pets, and it may encourage owners to switch to homemade or other alternative diets that are often nutritionally inferior.26–31
The most clearly beneficial chemicals on the blacklist are the preservatives. Preventing spoilage, pathogen growth, and loss of nutrients in pet food is critical to providing healthy diets. In the absence of convincing evidence that commonly used and legally approved preservatives are actually harmful, removing them can only lead to less safe and healthy food for pets.
The evidence of health risks for most of the additives on the list is weak and based primarily on in vitroand lab animal studies that do not reliably predict the effects of normal use in pet foods. Most of these additives have been used for decades and reviewed periodically by regulators with no convincing evidence of negative health effects in humans or pets. Some may have risks that warrant removing them from use, but the evidence to make this case is lacking.
One can make the argument, of course, that any substance which has shown any hint of toxicity in lab animal studies ought to be avoided. There is little evidence, however, that this precautionary approach actually reduces harm. If the substances that are abandoned are actually safe, then there is no benefit. And there is always the potential that new, less thoroughly tested alternatives may have greater risks, even if they are “natural.”32
There is even evidence that some of the additives on the Petco list may actually have health benefits (see references from Table 1). Many have antiseptic, anti-inflammatory, anti-neoplastic, and antioxidant activity or other potentially beneficial uses. While the evidence for these effects is weak and based mostly on in vitroand lab animal studies, this is no less convincing than the evidence for negative health effects Petco has used to justify banning these compounds.
Irrational and unscientific reasoning is not likely to lead to good healthcare choices. Unjustified fear of grains has led to grain-free diets making up about 25% of the dog food market. There is no reason to believe these diets have health benefits, and there are beginning to be signs that feeding these diets may be harming dogs.33–35The same reasoning that underlies this blacklist has also led Petco to sell raw diets, which have well-established health risks,28,36–42and to offer worthless homeopathic remedies43–47that pet owners may mistakenly substitute for effective, science-based medical treatment.
The best way to protect our pets’ health is to rely on sound scientific evidence to help us weigh the risks and benefits of the food and medicine we provide, not to cater to irrational fears like chemophobia and meaningless distinctions such as “natural” and artificial.” Table 1 provides a partial list of the sources, regulatory approvals, and evidence for safety and potentially beneficial effects of the items on the Petco blacklist. This is not a comprehensive review, simply an illustration that the items on this list are often “natural,” are judge by government experts around the world to be safe as used in food for humans and animals, and may have beneficial uses that offset any risk they may present.
Veterinarians have a responsibility to support and educate pet owners and to challenge unscientific, fear-based marketing ploys like the Petco blacklist. The movement towards dangerous “natural” practices like feeding raw diets and avoiding vaccination is a real threat to animal welfare, and it is exacerbated by companies seeking market advantage through feeding and capitalizing on misconceptions and fear.
References
1. World Health Organization. Drowning.; 2018. https://www.who.int/news-room/fact-sheets/detail/drowning. Accessed December 24, 2018.
2. DHMO.ORG. Facts About Dihydrogen Monoxide. http://www.dhmo.org/facts.html. Published 2018. Accessed December 24, 2018.
3. DHMO.ORG Research Division. DHMO Research Reports. http://www.dhmo.org/research.html. Published 2008. Accessed December 24, 2018.
4. Stuff.co.nz. National MP falls victim to water hoax. Stuff. http://www.stuff.co.nz/national/politics/38005/National-MP-falls-victim-to-water-hoax. Published 2009. Accessed December 24, 2018.
5. Robson D. Chemonoia: The fear blinding our minds to real dangers. BBC FUture.
6. Francl M. How to counteract chemophobia. Nat Chem. 2013;5(6):439-440. doi:10.1038/nchem.1661
7. Ropeik D. On the roots of, and solutions to, the persistent battle between “chemonoia” and rationalist denialism of the subjective nature of human cognition. Hum Exp Toxicol. 2015;34(12):1272-1278. doi:10.1177/0960327115603592
8. Petco. Petco First and Only Major Retailer of Pet Food to Not Sell Food and Treats with Artificial Ingredients. PR Newswire. https://www.prnewswire.com/news-releases/petco-first-and-only-major-retailer-of-pet-food-to-not-sell-food-and-treats-with-artificial-ingredients-300748831.html. Published 2018. Accessed December 24, 2018.
9. Ingredients Which Do Not Meet Petco’s New Nutritional Standards. https://www.petco.com/shop/en/petcostore/c/betternutrition-ingredients. Published 2018. Accessed December 24, 2018.
10. Bastaki M, Farrell T, Bhusari S, Bi X, Scrafford C. Estimated daily intake and safety of FD&C food-colour additives in the US population. Food Addit Contam Part A. 2017;34(6):891-904. doi:10.1080/19440049.2017.1308018
11. Bastaki M, Farrell T, Bhusari S, Pant K, Kulkarni R. Lack of genotoxicity in vivo for food color additive Allura Red AC. Food Chem Toxicol. 2017;105:308-314. doi:10.1016/j.fct.2017.04.037
12. Bastaki M, Farrell T, Bhusari S, Pant K, Kulkarni R. Lack of genotoxicity in vivo for food color additive Tartrazine. Food Chem Toxicol. 2017;105:278-284. doi:10.1016/j.fct.2017.04.034
13. Amchova P, Kotolova H, Ruda-Kucerova J. Health safety issues of synthetic food colorants. Regul Toxicol Pharmacol. 2015;73(3):914-922. doi:10.1016/j.yrtph.2015.09.026
14. Oplatowska-Stachowiak M, Elliott CT. Food colors: Existing and emerging food safety concerns. Crit Rev Food Sci Nutr. 2017;57(3):524-548. doi:10.1080/10408398.2014.889652
15. Younes M, Aggett P, Aguilar F, et al. Evaluation of four new studies on the potential toxicity of titanium dioxide used as a food additive (E 171). EFSA J. 2018;16(7). doi:10.2903/j.efsa.2018.5366
16. European Food Safety Authority (EFSA). Food colours: titanium dioxide marks re-evaluation milestone |. http://www.efsa.europa.eu/en/press/news/160914. Published 2016. Accessed December 25, 2018.
17. Food and Drug Administration (FDA). Titanium Dioxide.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=73.575. Accessed December 25, 2018.
18. European Food Safety Authority (EFSA). Food Colours. http://www.efsa.europa.eu/en/topics/topic/food-colours. Published 2016. Accessed December 25, 2018.
19. Food Standards Australia New Zealand. Food Colours. http://www.foodstandards.gov.au/consumer/additives/foodcolour/pages/default.aspx. Published 2012. Accessed December 25, 2018.
20. Food Standards Australia New Zealand (FSANZ). Supplementary food colours report. http://www.foodstandards.gov.au/science/surveillance/pages/supplementaryfoodcol5571.aspx. Published 2012. Accessed December 25, 2018.
21. FDA. Overview of Food Ingredients, Additives & Colors. US Food Drug Adm. 2010. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm094211.htm#qahyper. Accessed December 25, 2018.
22. Center for Food Safety and Applied Nutrition F. Food Additives & Ingredients – Color Additives Questions and Answers for Consumers. https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm488219.htm. Accessed December 25, 2018.
23. Laflamme D, Izquierdo O, Eirmann L, Binder S. Myths and Misperceptions About Ingredients Used in Commercial Pet Foods. Vet Clin North Am Small Anim Pract. 2014;44(4):689-698. doi:10.1016/j.cvsm.2014.03.002
24. Wortinger A. Nutritional Myths. J Am Anim Hosp Assoc. 2005;41(4):273-276. doi:10.5326/0410273
25. Case LP, Daristotle L, Hayek L, Foess Raasch M. Common Nutrition Myths and Feeding Practices. In: Canine and Feline Nutrition. 3rd ed. Maryland Hwights, MO: Mosby Elsevier; 2010:277-294.
26. Weeth LP. Focus on nutrition: Home-prepared diets for dogs and cats. Compend Contin Educ Vet. 2013;35(3):E3. http://www.ncbi.nlm.nih.gov/pubmed/23532921. Accessed October 28, 2018.
27. Streiff EL, Zwischenberger B, Butterwick RF, Wagner E, Iben C, Bauer JE. A Comparison of the Nutritional Adequacy of Home-Prepared and Commercial Diets for Dogs. J Nutr. 2002;132(6):1698S-1700S. doi:10.1093/jn/132.6.1698S
28. Schlesinger DP, Joffe DJ. Raw food diets in companion animals: a critical review. Can Vet J = La Rev Vet Can. 2011;52(1):50-54. http://www.ncbi.nlm.nih.gov/pubmed/21461207. Accessed October 28, 2018.
29. Streiff EL, Zwischenberger B, Butterwick RF, Wagner E, Iben C, Bauer JE. A comparison of the nutritional adequacy of home-prepared and commercial diets for dogs. J Nutr. 2002;132(6 Suppl 2):1698S-700S. doi:10.1093/jn/132.6.1698S
30. Stockman J, Fascetti AJ, Kass PH, Larsen JA. Evaluation of recipes of home-prepared maintenance diets for dogs. J Am Vet Med Assoc. 2013;242(11):1500-1505. doi:10.2460/javma.242.11.1500
31. Hutchinson D, Freeman LM, McCarthy R, Anastasio J, Shaw SP, Sutherland-Smith J. Seizures and severe nutrient deficiencies in a puppy fed a homemade diet. J Am Vet Med Assoc. 2012;241(4):477-483. doi:10.2460/javma.241.4.477
32. Simon JE, Decker EA, Ferruzzi MG, et al. Establishing Standards on Colors from Natural Sources. J Food Sci. 2017;82(11):2539-2553. doi:10.1111/1750-3841.13927
33. Freeman LM, Stern JA, Fries R, Adin DB, Rush JE. Diet-associated dilated cardiomyopathy in dogs: what do we know? J Am Vet Med Assoc. 2018;253(11):1390-1394. doi:10.2460/javma.253.11.1390
34. Kaplan JL, Stern JA, Fascetti AJ, et al. Taurine deficiency and dilated cardiomyopathy in golden retrievers fed commercial diets. Loor JJ, ed. PLoS One. 2018;13(12):e0209112. doi:10.1371/journal.pone.0209112
35. Adin D, DeFrancesco TC, Keene B, et al. Echocardiographic phenotype of canine dilated cardiomyopathy differs based on diet type. J Vet Cardiol. 2019;21:1-9. doi:10.1016/J.JVC.2018.11.002
36. Freeman LM, Chandler ML, Hamper BA, Weeth LP. Current knowledge about the risks and benefits of raw meat-based diets for dogs and cats. J Am Vet Med Assoc. 2013;243(11):1549-1558. doi:10.2460/javma.243.11.1549
37. Health England P. Investigation into an Outbreak of Shiga Toxin Producing Escherichia Coli.; 2017. www.facebook.com/PublicHealthEngland. Accessed December 6, 2018.
38. Chengappa MM, Staats J, Oberst RD, Gabbert NH, McVey S. Prevalence of Salmonellain Raw Meat used in Diets of Racing Greyhounds. J Vet Diagnostic Investig. 1993;5(3):372-377. doi:10.1177/104063879300500312
39. Finley R, Ribble C, Aramini J, et al. The risk of salmonellae shedding by dogs fed Salmonella-contaminated commercial raw food diets. Can Vet J = La Rev Vet Can. 2007;48(1):69-75. http://www.ncbi.nlm.nih.gov/pubmed/17310625. Accessed October 27, 2018.
40. Joffe DJ, Schlesinger DP. Preliminary assessment of the risk of Salmonella infection in dogs fed raw chicken diets. Can Vet J = La Rev Vet Can. 2002;43(6):441-442. http://www.ncbi.nlm.nih.gov/pubmed/12058569. Accessed October 27, 2018.
41. Weese JS, Rousseau J, Arroyo L. Bacteriological evaluation of commercial canine and feline raw diets. Can Vet J = La Rev Vet Can. 2005;46(6):513-516. http://www.ncbi.nlm.nih.gov/pubmed/16048011. Accessed October 27, 2018.
42. Strohmeyer RA, Morley PS, Hyatt DR, Dargatz DA, Scorza AV, Lappin MR. Evaluation of bacterial and protozoal contamination of commercially available raw meat diets for dogs. J Am Vet Med Assoc. 2006;228(4):537-542. doi:10.2460/javma.228.4.537
43. Ernst E. Homeopathy – The Undiluted Facts. Cham: Springer International Publishing; 2016. doi:10.1007/978-3-319-43592-3
44. Ernst E. A systematic review of systematic reviews of homeopathy. Br J Clin Pharmacol. 2002;54(6):577-582. http://www.ncbi.nlm.nih.gov/pubmed/12492603. Accessed November 12, 2018.
45. Shang A, Huwiler-Müntener K, Nartey L, et al. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet (London, England). 2005;366(9487):726-732. doi:10.1016/S0140-6736(05)67177-2
46. Ernst E, Pittler MH. Re-analysis of previous meta-analysis of clinical trials of homeopathy. J Clin Epidemiol. 2000;53(11):1188. http://www.ncbi.nlm.nih.gov/pubmed/11186614. Accessed November 12, 2018.
47. Ernst E. Classical homoeopathy versus conventional treatments: a systematic review. 1999. https://www.ncbi.nlm.nih.gov/books/NBK67846/. Accessed November 12, 2018.
48. Feron VJ, Til HP, de Vrijer F, Woutersen RA, Cassee FR, van Bladeren PJ. Aldehydes: occurrence, carcinogenic potential, mechanism of action and risk assessment. Mutat Res. 259(3-4):363-385. http://www.ncbi.nlm.nih.gov/pubmed/2017217. Accessed December 25, 2018.
49. ACETALDEHYDE WORKING GROUP Comments on the CLH Report on Acetaldehyde; Proposal for Harmonised Classification and Labeling (June 2015). https://echa.europa.eu/documents/10162/b9885f2c-b491-4ad4-8900-8cba349b15a0. Accessed December 25, 2018.
50. Evaluation Report of Food Additives Acetaldehyde Food Safety Commission Evaluation Results on the Health Risk Assessment of Acetaldehyde as Food Additive.; 2005. http://www.fsc.go.jp/english/evaluationreports/foodadditive/acetaldehyde_report.pdf. Accessed December 25, 2018.
51. Food and Drug Administration (FDA). Substances Generally Recognized as Safe.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=182.60. Accessed December 25, 2018.
52. World Health Organization (WHO). Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). http://apps.who.int/food-additives-contaminants-jecfa-database/search.aspx. Published 2017. Accessed December 25, 2018.
53. V. V. The Asphyxiating and Exsanguinating Death of President George Washington. Perm J. 2004;8(2):2002-2005.
54. Aguilar F, Nybro Autrup H, Barlow S, et al. Flavouring Group Evaluation 11, Revision 1 (FGE.11Rev1) 1 Aliphatic Dialcohols, Diketones, and Hydroxyketones from Chemical Group 10 Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) PANEL MEMBERS. Vol 493.; 2008. http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/493.pdf. Accessed December 25, 2018.
55. Xiao Z, Lu JR. Generation of Acetoin and Its Derivatives in Foods. 2014. doi:10.1021/jf5013902
56. Almodares A, Ranjbar M, Hadi MR. Effects of nitrogen treatments and harvesting stages on the aconitic acid, invert sugar and fiber in sweet sorghum cultivars. J Environ Biol. 2010;31(6):1001-1005. http://www.ncbi.nlm.nih.gov/pubmed/21506489. Accessed December 25, 2018.
57. Food and Drug Administration (FDA). Aconitic Acid.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1007. Accessed December 25, 2018.
58. Aprotosoaie AC, Costache I-I, Miron A. Anethole and Its Role in Chronic Diseases. In: Advances in Experimental Medicine and Biology. Vol 929. ; 2016:247-267. doi:10.1007/978-3-319-41342-6_11
59. Aquilina G, Bories G, Chesson A, et al. Scientific Opinion on the safety and efficacy of allylhydroxybenzenes (chemical group 18) when used as flavourings for all animal species 1 EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA J. 2011;9(12):2440. doi:10.2903/j.efsa.2011.2440
60. Kim KY, Lee HS, Seol GH. Anti-inflammatory effects of trans -anethole in a mouse model of chronic obstructive pulmonary disease. Biomed Pharmacother. 2017;91:925-930. doi:10.1016/j.biopha.2017.05.032
61. Scott HR, Scott LE. Process of treating nut kernels to produce food ingredients. July 1920. https://patents.google.com/patent/US1416128. Accessed December 25, 2018.
62. Adams TB, Cohen SM, Doull J, et al. The FEMA GRAS assessment of benzyl derivatives used as flavor ingredients. Food Chem Toxicol. 2005;43(8):1207-1240. doi:10.1016/J.FCT.2004.11.014
63. da Silva DS, da Silva CEH, Soares MSP, et al. Thiazolidin-4-ones from 4-(methylthio)benzaldehyde and 4-(methylsulfonyl)benzaldehyde: Synthesis, antiglioma activity and cytotoxicity. Eur J Med Chem. 2016;124:574-582. doi:10.1016/j.ejmech.2016.08.057
64. Jang TY, Park C-S, Kim K-S, Heo M-J, Kim YH. Benzaldehyde suppresses murine allergic asthma and rhinitis. Int Immunopharmacol. 2014;22(2):444-450. doi:10.1016/j.intimp.2014.07.029
65. Final Report on the Safety Assessment of Benzaldehyde1. Int J Toxicol. 2006;25(1_suppl):11-27. doi:10.1080/10915810600716612
66. Heidor R, Ortega JF, de Conti A, Ong TP, Moreno FS. Anticarcinogenic actions of tributyrin, a butyric acid prodrug. Curr Drug Targets. 2012;13(14):1720-1729. http://www.ncbi.nlm.nih.gov/pubmed/23140283. Accessed December 25, 2018.
67. Sossai P. Butyric acid: what is the future for this old substance? Swiss Med Wkly. 2012;142:w13596. doi:10.4414/smw.2012.13596
68. Manrique Vergara D, González Sánchez ME. Ácidos grasos de cadena corta (ácido butírico) y patologías intestinales. Nutr Hosp. 2017;34(4):58-61. doi:10.20960/nh.1573
69. McNabney SM, Henagan TM. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients. 2017;9(12). doi:10.3390/nu9121348
70. Ayd?n E, Türkez H, Kele? MS. Potential anticancer activity of carvone in N2a neuroblastoma cell line. Toxicol Ind Health. 2015;31(8):764-772. doi:10.1177/0748233713484660
71. Souza FVM, da Rocha MB, de Souza DP, Marçal RM. (?)-Carvone: Antispasmodic effect and mode of action. Fitoterapia. 2013;85:20-24. doi:10.1016/j.fitote.2012.10.012
72. Muruganathan U, Srinivasan S. Beneficial effect of carvone, a dietary monoterpene ameliorates hyperglycemia by regulating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2016;84:1558-1567. doi:10.1016/j.biopha.2016.11.025
73. Nogoceke FP, Barcaro IMR, de Sousa DP, Andreatini R. Antimanic-like effects of (R)-(?)-carvone and (S)-(+)-carvone in mice. Neurosci Lett. 2016;619:43-48. doi:10.1016/j.neulet.2016.03.013
74. de Carvalho CCCR, da Fonseca MMR. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006;95(3):413-422. doi:10.1016/J.FOODCHEM.2005.01.003
75. Zhu R, Liu H, Liu C, et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol Res. 2017;122:78-89. doi:10.1016/j.phrs.2017.05.019
76. Chen B-J, Fu C-S, Li G-H, et al. Cinnamaldehyde Analogues as Potential Therapeutic Agents. Mini Rev Med Chem. 2017;17(1):33-43. http://www.ncbi.nlm.nih.gov/pubmed/26791737. Accessed December 25, 2018.
77. Friedman M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J Agric Food Chem. 2017;65(48):10406-10423. doi:10.1021/acs.jafc.7b04344
78. Shreaz S, Wani WA, Behbehani JM, et al. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia. 2016;112:116-131. doi:10.1016/j.fitote.2016.05.016
79. Pérez Zamora C, Torres C, Nuñez M. Antimicrobial Activity and Chemical Composition of Essential Oils from Verbenaceae Species Growing in South America. Molecules. 2018;23(3):544. doi:10.3390/molecules23030544
80. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a Request from the Commission Related to Flavouring Group Evaluation 23: Aliphatic, Alicyclic and Aromatic Ethers Including Anisole Derivatives From Chemical Groups 15, 16 and 26 (Commission Regulation (EC) No.; 1565. http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/417.pdf. Accessed December 25, 2018.
81. Patel S. Plant essential oils and allied volatile fractions as multifunctional additives in meat and fish-based food products: a review. Food Addit Contam Part A. 2015;32(7):1049-1064. doi:10.1080/19440049.2015.1040081
82. Liu K, Chen Q, Liu Y, Zhou X, Wang X. Isolation and Biological Activities of Decanal, Linalool, Valencene, and Octanal from Sweet Orange Oil. J Food Sci. 2012;77(11):C1156-C1161. doi:10.1111/j.1750-3841.2012.02924.x
83. Laribi B, Kouki K, M’Hamdi M, Bettaieb T. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia. 2015;103:9-26. doi:10.1016/j.fitote.2015.03.012
84. EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Scientific Opinion on the safety and efficacy of straight-chain primary aliphatic alcohols/aldehydes/acids, acetals and esters with esters containing saturated alcohols and acetals containing saturated aldehydes (chemical group 1) when used as flavourings. EFSA J. 2013;11(4):3169.
85. Chhikara N, Kour R, Jaglan S, Gupta P, Gat Y, Panghal A. Citrus medica: nutritional, phytochemical composition and health benefits – a review. Food Funct. 2018;9(4):1978-1992. doi:10.1039/c7fo02035j
86. Dunnington D, Butterworth KR, Gaunt IF, Mason PL, Evans JG, Gangolli SD. Long-term toxicity study of ethyl methylphenylglycidate (strawberry aldehyde) in the rat. Food Cosmet Toxicol. 1981;19:691-699. doi:10.1016/0015-6264(81)90522-8
87. Khalil AA, Rahman U ur, Khan MR, Sahar A, Mehmood T, Khan M. Essential oil eugenol: sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017;7(52):32669-32681. doi:10.1039/C7RA04803C
88. Flavouring Group Evaluation 60 (FGE.60) 1?: Consideration of Eugenol and Related Hydroxyallylbenzene Derivatives Evaluated by JECFA (65 Th Meeting) Structurally Related to Ring-Substituted Phenolic Substances Evaluated by EFSA in FGE.22 (2006) Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC). http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1178620772628.htm. Accessed December 25, 2018.
89. Hu Q, Zhou M, wei S. Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field. J Food Sci. 2018;83(6):1476-1483. doi:10.1111/1750-3841.14180
90. Api AM, Belsito D, Bhatia S, et al. RIFM fragrance ingredient safety assessment, Eugenol, CAS Registry Number 97-53-0. Food Chem Toxicol. 2016;97:S25-S37. doi:10.1016/j.fct.2015.12.013
91. Marchese A, Barbieri R, Coppo E, et al. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit Rev Microbiol. 2017;43(6):668-689. doi:10.1080/1040841X.2017.1295225
92. Fujisawa S, Murakami Y. Eugenol and Its Role in Chronic Diseases. In: Advances in Experimental Medicine and Biology. Vol 929. ; 2016:45-66. doi:10.1007/978-3-319-41342-6_3
93. Food and Drug Administration. CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1257. Accessed December 25, 2018.
94. Fragrance raw materials monographs: Geranyl acetate. Food Cosmet Toxicol. 1974;12(7-8):885-886. doi:10.1016/0015-6264(74)90167-9
95. Seebaluck R, Gurib-Fakim A, Mahomoodally F. Medicinal plants from the genus Acalypha (Euphorbiaceae)–A review of their ethnopharmacology and phytochemistry. J Ethnopharmacol. 2015;159:137-157. doi:10.1016/j.jep.2014.10.040
96. Qi F, Yan Q, Zheng Z, Liu J, Chen Y, Zhang G. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J BUON. 23(2):346-352. http://www.ncbi.nlm.nih.gov/pubmed/29745075. Accessed December 25, 2018.
97. National Toxicology Program. NTP Carcinogenesis Studies of Food Grade Geranyl Acetate (71% Geranyl Acetate, 29% Citronellyl Acetate) (CAS No. 105-87-3) in F344/N Rats and B6C3F1 Mice (Gavage Study). Natl Toxicol Program Tech Rep Ser. 1987;252:1-162. http://www.ncbi.nlm.nih.gov/pubmed/12748693. Accessed December 25, 2018.
98. Heidor R, Ortega JF, de Conti A, Ong TP, Moreno FS. Anticarcinogenic actions of tributyrin, a butyric acid prodrug. Curr Drug Targets. 2012;13(14):1720-1729. http://www.ncbi.nlm.nih.gov/pubmed/23140283. Accessed December 26, 2018.
99. Bedford A, Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr. 2018;4(2):151-159. doi:10.1016/J.ANINU.2017.08.010
100. Kuefer R, Hofer MD, Altug V, et al. Sodium butyrate and tributyrin induce in vivo growth inhibition and apoptosis in human prostate cancer. Br J Cancer. 2004;90(2):535-541. doi:10.1038/sj.bjc.6601510
101. Kang SN, Lee E, Lee M-K, Lim S-J. Preparation and evaluation of tributyrin emulsion as a potent anti-cancer agent against melanoma. Drug Deliv. 2011;18(2):143-149. doi:10.3109/10717544.2010.522610
102. Cresci GA, Glueck B, McMullen MR, Xin W, Allende D, Nagy LE. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J Gastroenterol Hepatol. 2017;32(9):1587-1597. doi:10.1111/jgh.13731
103. Aquilina G, Bach A, Bampidis V, et al. Scientific Opinion on the safety and efficacy of branched-chain primary aliphatic alcohols/aldehydes/acids, acetals and esters with esters containing branched-chain alcohols and acetals containing branched-chain aldehydes (chemical group 2) when used as flavourings for all animal species 1 EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). EFSA J. 2012;10(10):2927. doi:10.2903/j.efsa.2012.2927
104. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21. 21CFR184.1903; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1903. Accessed December 26, 2018.
105. Sun J. D-Limonene: safety and clinical applications. Altern Med Rev. 2007;12(3):259-264. http://www.ncbi.nlm.nih.gov/pubmed/18072821. Accessed December 26, 2018.
106. Vieira AJ, Beserra FP, Souza MC, Totti BM, Rozza AL. Limonene: Aroma of innovation in health and disease. Chem Biol Interact. 2018;283:97-106. doi:10.1016/j.cbi.2018.02.007
107. Ravichandran C, Badgujar PC, Gundev P, Upadhyay A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem Toxicol. 2018;120:668-680. doi:10.1016/j.fct.2018.07.052
108. Kim YW, Kim MJ, Chung BY, et al. Safety evaluation and risk assessment of D-limonene. J Toxicol Environ Heal – Part B Crit Rev. 2013;16(1):17-38. doi:10.1080/10937404.2013.769418
109. Scientific Opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical group 31) when used as flavourings for all animal species; Scientific Opinion on the safety and efficacy of aliphatic and aromatic hydrocarbons (chemical group 31) when used as flavourings for all animal species. EFSA J. 2015;13(3):4053. doi:10.2903/j.efsa.2015.4053
110. Pereira I, Severino P, Santos AC, Silva AM, Souto EB. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surfaces B Biointerfaces. 2018;171:566-578. doi:10.1016/j.colsurfb.2018.08.001
111. Seol G-H, Kang P, Lee HS, Seol GH. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurol. 2016;16(1):17. doi:10.1186/s12883-016-0541-3
112. de Oliveira Lima MI, Araújo de Medeiros AC, Souza Silva KV, Cardoso GN, de Oliveira Lima E, de Oliveira Pereira F. Investigation of the antifungal potential of linalool against clinical isolates of fluconazole resistant Trichophyton rubrum. J Mycol Med. 2017;27(2):195-202. doi:10.1016/j.mycmed.2017.01.011
113. Iwasaki K, Zheng Y-W, Murata S, et al. Anticancer effect of linalool viacancer-specific hydroxyl radical generation in human colon cancer. World J Gastroenterol. 2016;22(44):9765. doi:10.3748/wjg.v22.i44.9765
114. Herman A, Tambor K, Herman A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr Microbiol. 2016;72(2):165-172. doi:10.1007/s00284-015-0933-4
115. Scientific Opinion on the safety and efficacy of aliphatic, alicyclic and aromatic saturated and unsaturated tertiary alcohols and esters with esters containing tertiary alcohols ethers (chemical group 6) when used as flavourings for all animal species. EFSA J. 2012;10(11):2966. doi:10.2903/j.efsa.2012.2966
116. Rombolà L, Amantea D, Russo R, et al. Rational Basis for the Use of Bergamot Essential Oil in Complementary Medicine to Treat Chronic Pain. Mini Rev Med Chem. 2016;16(9):721-728. http://www.ncbi.nlm.nih.gov/pubmed/26996621. Accessed December 26, 2018.
117. Koulivand PH, Khaleghi Ghadiri M, Gorji A. Lavender and the nervous system. Evid Based Complement Alternat Med. 2013;2013:681304. doi:10.1155/2013/681304
118. National Center for Biotechnology Information. Malic Acid. PubChem Compound Database.
119. Abraham GE, Flechas JD. Management of Fibromyalgia: Rationale for the Use of Magnesium and Malic Acid. J Nutr Med. 1992;3(1):49-59. doi:10.3109/13590849208997961
120. Tyka AK, Chwastowski M, Cison T, et al. Effect of creatine malate supplementation on physical performance, body composition and selected hormone levels in spinters and long-distance runners. Acta Physiol Hung. 2015;102(1):114-122. doi:10.1556/APhysiol.102.2015.1.12
121. Rodgers AL, Webber D, de Charmoy R, Jackson GE, Ravenscroft N. Malic Acid Supplementation Increases Urinary Citrate Excretion and Urinary pH: Implications for the Potential Treatment of Calcium Oxalate Stone Disease. J Endourol. 2014;28(2):229-236. doi:10.1089/end.2013.0477
122. National Center for Biotechnology Information. Methyl Anthranilate. PubChem Database.
123. Askham LR. Proceedings – Vertebrate Pest Conference. In: Proceedings… Vertebrate Pest Conference (USA). University of California, Davis; 1992. http://agris.fao.org/agris-search/search.do?recordID=US9416552. Accessed December 26, 2018.
124. Cummings JL, Avery ML, Pochop PA, et al. Evaluation of a methyl anthranilate formulation for reducing bird damage to blueberries. Crop Prot. 1995;14(3):257-259. doi:10.1016/0261-2194(95)00016-F
125. Aquilina G, Bories G, Chesson A, et al. Scientific Opinion on the safety and efficacy of anthranilate derivatives (chemical group 27) when used as flavourings for all animal species 1 EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) Anthranilate derivatives (CG 27) for all species. EFSA J. 2011;9(12):2441. doi:10.2903/j.efsa.2011.2441
126. Rao VRS, Raju SS, Sarma VU, et al. Simultaneous determination of bioactive compounds in Piper nigrum L. and a species comparison study using HPLC-PDA. Nat Prod Res. 2011;25(13):1288-1294. doi:10.1080/14786419.2010.535158
127. Li X, Choi Y, Yanakawa Y, Park T. Piperonal prevents high-fat diet-induced hepatic steatosis and insulin resistance in mice via activation of adiponectin/AMPK pathway. Int J Obes. 2014;38(1):140-147. doi:10.1038/ijo.2013.70
128. Chu S, Narayan VP, Sung M-K, Park T. Piperonal attenuates visceral adiposity in mice fed a high-fat diet: potential involvement of the adenylate cyclase-protein kinase A dependent pathway. Mol Nutr Food Res. 2017;61(11):1601124. doi:10.1002/mnfr.201601124
129. Meriga B, Parim B, Chunduri VR, et al. Antiobesity potential of Piperonal: promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr Metab (Lond). 2017;14(1):72. doi:10.1186/s12986-017-0228-9
130. Anantharaju PG, Gowda PC, Vimalambike MG, Madhunapantula S V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J. 2016;15(1):99. doi:10.1186/s12937-016-0217-2
131. del Olmo A, Calzada J, Nuñez M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit Rev Food Sci Nutr. 2017;57(14):3084-3103. doi:10.1080/10408398.2015.1087964
132. Aguilar F, Crebelli R, Domenico A Di, et al. Scientific Opinion on the re-evaluation of benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) as food additives; Scientific Opinion on the re-evaluation of benzoic acid (E 210), sodium benzoate (E 211), potassium benzoate (E 212) and calcium benzoate (E 213) as food additives. EFSA J. 2016;14(3):110. doi:10.2903/j.efsa.2016.4433
133. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1021. Accessed December 26, 2018.
134. Qualley A V., Widhalm JR, Adebesin F, Kish CM, Dudareva N. Completion of the core -oxidative pathway of benzoic acid biosynthesis in plants. Proc Natl Acad Sci. 2012;109(40):16383-16388. doi:10.1073/pnas.1211001109
135. Andersen FA. Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. In:International Journal of Toxicology. Vol 20. ; 2001:23-50. doi:10.1080/10915810152630729
136. Food Standards Authority Australia New Zealand (FSANZ). SCHEDULE 1 Permitted Uses of Food Additives by Food Type. http://www.foodstandards.gov.au/code/Documents/standard_1_3_1_additives_vol_2_v1321.pdf. Accessed December 27, 2018.
137. Fidler MC, Davidsson L, Zeder C, Hurrell RF. Erythorbic acid is a potent enhancer of nonheme-iron absorption.Am J Clin Nutr. 2004;79(1):99-102. doi:10.1093/ajcn/79.1.99
138. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3041. Accessed December 27, 2018.
139. Aguilar F, Crebelli R, Domenico A Di, et al. Scientific Opinion on the re-evaluation of erythorbic acid (E 315) and sodium erythorbate (E 316) as food additives; Scientific Opinion on the re-evaluation of erythorbic acid (E 315) and sodium erythorbate (E 316) as food additives. EFSA J. 2016;14(1):4360-4362. doi:10.2903/j.efsa.2016.4360
140. Miura K, Yazama F, Tai A. Oxidative stress-mediated antitumor activity of erythorbic acid in high doses. Biochem Biophys Reports. 2015;3:117-122. doi:10.1016/j.bbrep.2015.07.018
141. Scientific Opinion on the safety evaluation of the substance, thiodipropionic acid, ditetradecyl ester, CAS No. 16545-54-3, for use in food contact materials. EFSA J. 2011;9(4):2126. doi:10.2903/j.efsa.2011.2126
142. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3109. Accessed December 27, 2018.
143. Hocman G. Chemoprevention of cancer: phenolic antioxidants (BHT, BHA). Int J Biochem. 1988;20(7):639-651. http://www.ncbi.nlm.nih.gov/pubmed/3053283. Accessed December 27, 2018.
144. Laflamme D, Izquierdo O, Eirmann L, Binder S. Myths and Misperceptions About Ingredients Used in Commercial Pet Foods. Vet Clin North Am Small Anim Pract. 2014;44(4):689-698. doi:10.1016/j.cvsm.2014.03.002
145. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=182.3169. Accessed December 27, 2018.
146. Rychen G, Aquilina G, Azimonti G, et al. Safety and efficacy of butylated hydroxyanisole (BHA) as a feed additive for all animal species. EFSA J. 2018;16(3). doi:10.2903/j.efsa.2018.5215
147. Iverson F. Phenolic antioxidants: Health protection branch studies on butylated hydroxyanisole. Cancer Lett. 1995;93(1):49-54. doi:10.1016/0304-3835(95)03787-W
148. Kahl R, Kappus H. [Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E]. Z Lebensm Unters Forsch. 1993;196(4):329-338. http://www.ncbi.nlm.nih.gov/pubmed/8493816. Accessed December 27, 2018.
149. Williams GM, Iatropoulos MJ, Whysner J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem Toxicol. 37(9-10):1027-1038. http://www.ncbi.nlm.nih.gov/pubmed/10541460. Accessed December 27, 2018.
150. Hirose M, Yada H, Hakoi K, Takahashi S, Ito N. Modification of carcinogenesis by alpha-tocopherol, t-butylhydroquinone, propyl gallate and butylated hydroxytoluene in a rat multi-organ carcinogenesis model. Carcinogenesis. 1993;14(11):2359-2364. http://www.ncbi.nlm.nih.gov/pubmed/8242867. Accessed December 27, 2018.
151. Aguilar R, Crebelli B, Dusemund P, et al. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Scientific Opinion on the re-evaluation of Butylated hydroxytoluene BHT (E 321) as a food additive Scientific Opinion on the re-evaluation of butylated hydroxytoluene BHT (E 321) as a . EFSA J. 2012;10(3):2588-2590. doi:10.2903/j.efsa.2012.2588
152. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3225. Accessed December 27, 2018.
153. Dzanis DA. Safety of Ethoxyquin in Dog Foods. J Nutr. 1991;121(suppl_11):S163-S164. doi:10.1093/jn/121.suppl_11.S163
154. CABEL MC, WALDROUP PW, SHERMER WD, CALABOTTA DF. Effects of Ethoxyquin Feed Preservative and Peroxide Level on Broiler Performance. Poult Sci. 1988;67(12):1725-1730. doi:10.3382/ps.0671725
155. Zhu J, Carozzi VA, Reed N, et al. Ethoxyquin provides neuroprotection against cisplatin-induced neurotoxicity. Sci Rep. 2016;6(1):28861. doi:10.1038/srep28861
156. Safety and efficacy of ethoxyquin (6?ethoxy?1,2?dihydro?2,2,4?trimethylquinoline) for all animal species. EFSA J. 2015;13(11). doi:10.2903/j.efsa.2015.4272
157. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=573.380. Accessed December 27, 2018.
158. Zhu J, Chen W, Mi R, Zhou C, Reed N, Höke A. Ethoxyquin prevents chemotherapy-induced neurotoxicity via Hsp90 modulation. Ann Neurol. 2013;74(6):893-904. doi:10.1002/ana.24004
159. B?aszczyk A, Augustyniak A, Skolimowski J. Ethoxyquin: An Antioxidant Used in Animal Feed. Int J Food Sci. 2013;2013:1-12. doi:10.1155/2013/585931
160. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3280&SearchTerm=dilauryl thiodipropionate. Accessed December 27, 2018.
161. Sasseville D, Alfalah M, Lacroix J-P. “Parabenoia” Debunked, or “Who’s Afraid of Parabens?”. Dermatitis. 2015;26(6):254-259. doi:10.1097/DER.0000000000000147
162. Soni MG, Taylor SL, Greenberg NA, Burdock GA. Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol. 2002;40(10):1335-1373. http://www.ncbi.nlm.nih.gov/pubmed/12387298. Accessed December 27, 2018.
163. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a Request from the Commission Related to Para Hydroxybenzoates The Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials In.; 2004. http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/83.pdf. Accessed December 27, 2018.
164. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1670. Accessed December 27, 2018.
165. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1490&SearchTerm=methylparaben . Accessed December 27, 2018.
166. Scientific Opinion on the re?evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228) as food additives. EFSA J. 2016;14(4). doi:10.2903/j.efsa.2016.4438
167. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3637. Accessed December 27, 2018.
168. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3616. Accessed December 27, 2018.
169. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3766. Accessed December 27, 2018.
170. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3798. Accessed December 27, 2018.
171. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3739. Accessed December 27, 2018.
172. Beckman Sundh U, Binderup M-L, Bolognesi C, et al. Scientific Opinion on the safety evaluation… CEF); Sci EFSA J. 2013;11(4):3155. doi:10.2903/j.efsa.2013.3155
173. Zar T, Graeber C, Perazella MA. Reviews: Recognition, Treatment, and Prevention of Propylene Glycol Toxicity. Semin Dial. 2007;20(3):217-219. doi:10.1111/j.1525-139X.2007.00280.x
174. Tobe M, Furuya T, Kawasaki Y, et al. Six-month toxicity study of butylated hydroxyanisole in beagle dogs. Food Chem Toxicol. 24(10-11):1223-1228. http://www.ncbi.nlm.nih.gov/pubmed/3804124. Accessed December 27, 2018.
175. Weil CS, Woodside MD, Smyth HF, Carpenter CP. Results of feeding propylene glycol in the diet to dogs for two years. Food Cosmet Toxicol. 1971;9(4):479-490. doi:10.1016/0015-6264(71)90078-2
176. Agency for Toxic Substances and Disease Registry, Public Health Service USD of H and HS. TOXICOLOGICAL PROFILE FOR PROPYLENE GLYCOL. Atlanta, GA; 1997. https://www.atsdr.cdc.gov/toxprofiles/tp189.pdf. Accessed December 27, 2018.
177. Staples CA, Davis JW. An examination of the physical properties, fate, ecotoxicity and potential environmental risks for a series of propylene glycol ethers. Chemosphere. 2002;49(1):61-73. http://www.ncbi.nlm.nih.gov/pubmed/12243331. Accessed December 27, 2018.
178. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1666. Accessed December 27, 2018.
179. Bigner DR, Goff JP, Faust MA, Tyler HD, Horst RL. Comparison of Oral Sodium Compounds for the Correction of Acidosis. J Dairy Sci. 1997;80(9):2162-2166. doi:10.3168/jds.S0022-0302(97)76163-0
180. SCHULTZ LH. Treatment of ketosis in dairy cattle with sodium propionate. Cornell Vet. 1952;42(1):148-155. http://www.ncbi.nlm.nih.gov/pubmed/14905895. Accessed December 27, 2018.
181. Kishimoto Y, Wakabayashi S, Takeda H. Effects of intravenous injection and intraperitoneal continual administration of sodium propionate on serum cholesterol levels in rats. J Nutr Sci Vitaminol (Tokyo). 1995;41(1):73-81. http://www.ncbi.nlm.nih.gov/pubmed/7616328. Accessed December 27, 2018.
182. Wang J, Wei Z, Zhang X, Wang Y, Yang Z, Fu Y. Propionate Protects against Lipopolysaccharide-Induced Mastitis in Mice by Restoring Blood–Milk Barrier Disruption and Suppressing Inflammatory Response. Front Immunol. 2017;8. doi:10.3389/fimmu.2017.01108
183. Chambers ES, Byrne CS, Aspey K, et al. Acute oral sodium propionate supplementation raises resting energy expenditure and lipid oxidation in fasted humans. Diabetes, Obes Metab. 2018;20(4):1034-1039. doi:10.1111/dom.13159
184. Safari R, Hoseinifar SH, Kavandi M. Modulation of antioxidant defense and immune response in zebra fish (Danio rerio) using dietary sodium propionate. Fish Physiol Biochem. 2016;42(6):1733-1739. doi:10.1007/s10695-016-0253-z
185. Safety of the extension of use of sodium propionate (E 281) as a food additive. EFSA J. 2016;14(8). doi:10.2903/j.efsa.2016.4546
186. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1784. Accessed December 27, 2018.
187. Silva De Souza SM, Hirata R, Moreira LO, et al. Influence of stannous chloride on the adhesive properties of Corynebacterium diphtheriae strains. Int J Mol Med. 2003;12(4):657-661. http://www.ncbi.nlm.nih.gov/pubmed/12964050. Accessed December 27, 2018.
188. João-Souza SH, Bezerra SJC, de Freitas PM, et al. In situ evaluation of fluoride-, stannous- and polyphosphate-containing solutions against enamel erosion. J Dent. 2017;63:30-35. doi:10.1016/j.jdent.2017.05.014
189. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1845. Accessed December 27, 2018.
190. Younes M, Aggett P, Aguilar F, et al. Re?evaluation of stannous chloride (E 512) as food additive. EFSA J. 2018;16(6). doi:10.2903/j.efsa.2018.5295
191. Wang X-B, Cui H, Liu X, Du J-B. Sulfur dioxide: foe or friend for life? Histol Histopathol. 2017;32(12):1231-1238. doi:10.14670/HH-11-904
192. Oehha. Sulfur Dioxide in Dried Fruit, Interpretive Guidelines.; 2012. http://www.oehha.ca.gov/prop65/prop65_list/Newlist.html. Accessed December 27, 2018.
193. Freedman BJ. Sulphur dioxide in foods and beverages: its use as a preservative and its effect on asthma. Br J Dis Chest. 1980;74(2):128-134. http://www.ncbi.nlm.nih.gov/pubmed/7426352. Accessed December 27, 2018.
194. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3862. Accessed December 27, 2018.
195. Chen S, Zheng S, Liu Z, et al. Endogeous sulfur dioxide protects against oleic acid-induced acute lung injury in association with inhibition of oxidative stress in rats. Lab Investig. 2015;95(2):142-156. doi:10.1038/labinvest.2014.147
196. Wang L-F, Su S-W, Wang L, et al. Tert-butylhydroquinone ameliorates doxorubicin-induced cardiotoxicity by activating Nrf2 and inducing the expression of its target genes. Am J Transl Res. 2015;7(10):1724-1735. http://www.ncbi.nlm.nih.gov/pubmed/26692920. Accessed December 27, 2018.
197. Zeng X-P, Li X-J, Zhang Q-Y, et al. Tert-Butylhydroquinone Protects Liver Against Ischemia/Reperfusion Injury in Rats Through Nrf2-Activating Anti-Oxidative Activity. Transplant Proc. 2017;49(2):366-372. doi:10.1016/j.transproceed.2016.12.008
198. Zhou N-Q, Liu N, Li P, Ping S, Peng Q-S, Shi W-D. Tert-butylhydroquinone promotes angiogenesis and improves heart functions in rats after myocardial infarction. Clin Exp Hypertens. 2017;39(5):402-408. doi:10.1080/10641963.2016.1259322
199. Gharavi N, Haggarty S, El-Kadi AOS. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab. 2007;8(1):1-7. http://www.ncbi.nlm.nih.gov/pubmed/17266519. Accessed December 27, 2018.
200. van Esch GJ. Toxicology of tert-butylhydroquinone (TBHQ). Food Chem Toxicol. 1986;24(10-11):1063-1065. doi:10.1016/0278-6915(86)90289-9
201. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.185. Accessed December 27, 2018.
202. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to tertiary-Butylhydroquinone (TBHQ). EFSA J. 2004;2(10):84. doi:10.2903/j.efsa.2004.84
203. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=74.250. Accessed December 25, 2018.
204. Food and Drug Administration (FDA). CFR – Code of Federal Regulations Title 21.; 2018. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?fr=74.302. Accessed December 25, 2018.










Great article! I don’st have to be embarrassed that I have always fed PPP!
Very informative and confirms my suspicions. My cats want nothing but Purina Pro Plan – and I’ve always felt that was a good, reputable brand. So off to Petsmart for me. My cats don’t understand ‘marketing strategies’. Even if Petco’s intentions are good, they should not be able to tout misinformation and tell customers what they should and shouldn’t purchase.
All Petco did was a marketing ploy for their stores. They continue to sell these “harmful” products online. Thanks for the article. I was hoping you would address Petco this year.
Just found an additional blog that is in line with your thinking: https://www.petfoodindustry.com/blogs/10-debunking-pet-food-myths-and-misconceptions/post/7956-fake-news-petco-drops-pet-food-with-artificial-ingredients?utm_source=KnowledgeMarketing&utm_medium=email&utm_content=Pet%20eNews&utm_campaign=19_03_15_PetENews&eid=436668196&bid=2395203
Thank you for fighting the good science-based fight! I logged in to the website today to reorder some food and discovered the “we’ll be discontinuing this soon” banner and a ridiculous page about “setting a new standard for nutrition” or something. It’s disappointing to see a nationwide pet supply store contribute to the chemophobic hysteria out there.
Thanks for the article. I am so annoyed by this. I have a Petco in a 5-minute walking distance from my home. Like Sara’s cat above, my cats like Purina Pro Plan, and I’ve been buying it there. I felt it was a good brand, and both of my cats like it. Now, most flavors are gone. Thankfully, I found it in a small pet store that still has it – for now, so I am going to shop there from now on. I might mention this Petco’s marketing ploy to that store, maybe they’ll carry “non-natural” food to make it easier for them to compete with Petco.
The whole thing is ridiculous.
I’m hoping Petsmart/Chewy.com do not copy this ridiculous policy.
For concerned pet owners, smaller local pet stores generally will order your preferred brand for you, just make the request.
They should just be selling dog food that is available. They should not be making nutritional recommendations.
But, Sabra, the employees are all experts on the *best* pet foods, didn’t you know that?! 🙂
What truly has me shaking my head is that Petco is implying they have either done the research, published it in a peer reviewed journal, allowed criticism of how the studies have been formulated, and that these results are repeatable. The average consumer is probably not skeptical when they are used to stores they regularly purchase from.
^ I guess I’m not an average consumer for pet food. I’m skeptical of holistic food, natural food, raw food, supplements, and dangerous OTC flea/tick products and other “health” products sold in pet stores (and now, ridiculous store policies). Just point me to the normal cat food on the tiny shelf between the rows and rows of “boutique and designer” pet food.
When Rachel Ray and GNC et al get into the pet food industry, you know there is a problem!
You may want to consider joining this FB Group–they also have a group established JUST for veterinarians to join. They are a group of vets and breeders collecting data on nutritional DCM. They have a lot of data from affected dogs, testimonials, copies of studies from Tufts, UC Davis and others, and some very interesting data they’ve compiled on the diets these affected dogs were on at the time of diagnosis or death. Please consider it. Thanks!https://www.facebook.com/groups/TaurineDCM/
Thanks for the tip. I do follow a couple of FB groups on this topic (there are several), but I hadn’t seen this one yet.
Mary Lanese, thank you so much for that link!
Have you heard of this one? https://www.drharveys.com/
It never ends. I am so glad I went back to traditional veterinary medicine.
Yeah, and “Dr.” Harvey is a chiropractor, not a vet or veterinary nutritionists, so not an “expert” in any relevant way.
https://www.answerspetfood.com/index.html
Here’s another one. No mention of a vet or vet nutritionist. Nothing.
“These statements and products have not been evaluated by the FDA. They are not intended to diagnose, treat,cure, or prevent any disease condition. If your pet has a health concern or condition, consult a Veterinarian”
LOL
Great article! I don’t understand why Hill’s has acceded to this marketing ploy by reformulating their pet foods. Boo Hiss!!!!! The inmates have taken over the asylum.
Pingback: Book Review- The Forever Dog: Surprising New Science to Help Your Canine Companion Live Younger, Healthier, and Longer |