Aging involves physical changes over time, but time is not the primary driver of these changes. Large-breed dogs age faster than small breed dogs, and there is great individual variation in the manifestations of aging. A key lesson we have learned is that chronological age and biological age are not identical. While we can measure chronological age easily, knowing the biological age of an individual is more useful in predicting and mitigating the health effects of aging.
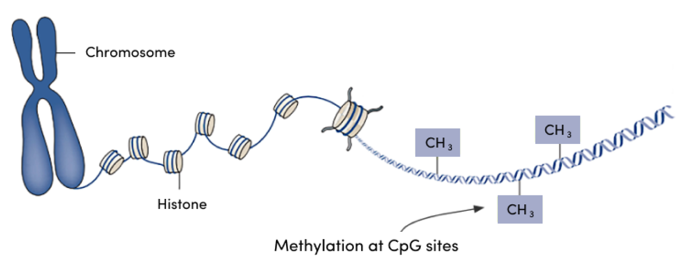
The extent of DNA methylation can serve as a measure of both chronological and biological age. Epigenetic clocks are measurements of DNA methylation at multiple sites which correlate with chronological age. This may not seem very useful since we often know chronological age directly. However, epigenetic clocks also accurately predict future mortality even when other risk factors for death and disease are accounted for. In this way, they can measure biological age as well. Such epigenetic clocks may help us measure aging and predict health outcomes as well as assess the impact of anti-aging treatments.
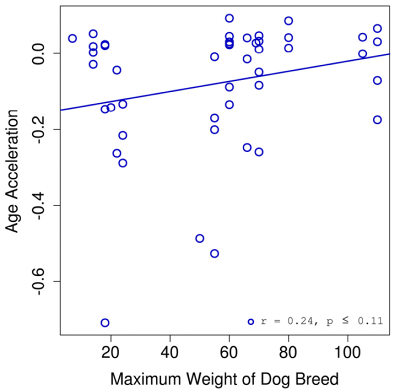
Epigenetic clocks have been developed for dogs, and they have given us further insight into patterns of aging within the species. One study** developed a clock for dogs and grey wolves that correlates strongly with chronological age. This clock also demonstrates that age acceleration (the difference between chronological and biological age) is greater for larger breed dogs, again showing that these dogs age faster (Figure 1).
**Thompson, M. J., von Holdt, B., Horvath, S., & Pellegrini, M. (2017). An epigenetic aging clock for dogs and wolves. Aging, 9(3), 1055–1068. https://doi.org/10.18632/aging.101211










so the more dna methylenation and telomere shorting they see the more aging? A chicken would have more dna methylenation and more telomere shorting than a macaw?
Let me ask it another way. You have two identical twins. The both have nearly identical telomeres and dna methylation at birth. You then measure them when they are 65. The twin with the longest telomeres and the one with the highest amount of dna methylation at the points measured would be expected to live the longest ?
These are statistical associations, so they don’t hold true for every comparison between individual animals or species. Birds are especially weird in terms of longevity and metabolism, so the associations have mostly been in mammals. That said, yes in general longer-lived species and individuals tend to have longer telomeres.
DNA methylation is a bit different. In general, there is more methylation over time, but it depends on which cog sites are tested and lots of other factors. DNA methylation again correlates in general with longevity and so can be a measure of biological age, so longer lived individuals may have different methylation patterns than shorter lived individuals, but it’s not an oracle that perfectly predicts the day you’ll drop dead. 🙂
The differences are not nearly that specific, so again it’s a general relationship not an oracle. Interesting experiment on this was the astronaut who showed some methylation and telomere changes compared to his identical twin after having been on the space station.