Here is a talk I gave at AVMA last week. Lot of folks attended, and lots of good followup questions!


Here is a talk I gave at AVMA last week. Lot of folks attended, and lots of good followup questions!

Last year, I participated in a research project evaluating risk factors for the diagnosis of osteoarthritis in dogs, using the large dataset in the Banfield medical records system. The research was published in November, 2023-
Graves JL, McKenzie BA, Koch Z, Naka A, Spofford N and Morrison J (2023) Body weight, gonadectomy, and other risk factors for diagnosis of osteoarthritis in companion dogs. Front Vet Sci 10:1275964.
This week, Dr. JoAnn Morrison from Banfield and I had the opportunity to present our research at the annual American Veterinary Medical Association convention. While this sort of research is not as drastic or “media-friendly” as reports of astonishing new discoveries, it is a critical foundation for understanding the health conditions our pets experience and how we can best identify and treat them or, ideally, prevent them.

RISK FACTORS FOR DEVELOPMENT OF OSTEOARTHRITIS IN COMPANION DOGS: RESULTS OF A LARGE RETROSPECTIVE STUDY
BASICS OF OSTEOARTHRITIS
Osteoarthritis (OA) is the most common joint disorder in dogs, and it contributes significantly to pain, disability, and ultimately euthanasia in aged dogs.1,2 The reported prevalence of OA varies between populations, and differences in methodology between studies make direct comparisons of these figures challenging. Prevalence of OA from 2.5% to over 80% have been reported, depending on factors such as the age of the population and how the condition is detected.1,3,4
The prevalence will be higher in older populations because OA is an incurable condition and so will be present and available for diagnosis longer as dogs age. It is also progressive and more likely to become clinically apparent over time. The condition is typically detected earlier and more frequently with radiographs compared with diagnosis based on clinical examination or symptoms, such as lameness. However, while radiographs are more sensitive, they may not accurately reflect the clinical significance of OA.5 By any measure, however, OA is a common condition with significant impact on quality of life.
Previous studies have reported a large and varied set of potential risk factors for OA.3 Age is the strongest and most consistent of these, reflecting the nature of OA as chronic, progressive, and an aging-associated condition. Higher body weight is also frequently identified as predictive of a higher risk of OA diagnosis. However, the relative importance of body size, body condition, or some interaction between the two, is not always clear. Both larger dogs and dogs with overweight or obese body condition appear to be at higher risk.3,6
Breed is another common risk factor, though again the contributions of body size, conformation, precipitating conditions (such as joint dysplasia or a predisposition to cranial cruciate ligament rupture), and genetics are often entangled. Neuter status, sex, activity, diet, and many other factors have been associated with OA risk in some studies. These associations are often of uncertain clinical relevance, and there are frequently conflicting findings from different studies.3,6
Complex interactions between multiple factors likely influence the development and severity of OA in any one dog. However, a clearer understanding of the main risk factors, especially those which could potentially be modified in individuals or altered through breeding in populations, would help veterinarians reduce the harmful impact of this condition on the welfare of companion dogs.
THE STUDY AND THE DATA
In this retrospective cohort study, Cox proportional hazard models were used to test for associations between osteoarthritis incidence and age at baseline, sex, maximum body weight, maximum body condition score, neuter status, and age at neutering. The same model was used to test these associations in 12 representative breeds, chosen based on breed weight and sample size.
THE RESULTS
Older age, higher adult body weight, gonadectomy, and younger age at gonadectomy were significantly associated with higher risks of osteoarthritis in the total cohort and in all 12 breeds evaluated. Higher body condition scores and sex were also significantly associated with osteoarthritis but with minimal effect sizes in the overall cohort, and these risk factors were not consistently significant in all breeds tested.
CLINICAL IMPLICATIONS
The results of this study confirm that OA is commonly diagnosed in companion dogs seen in primary care practice. The strongest risk factor by far was age, consistent with expectations based on previous research. Aging is currently not seen as a modifiable risk factor, but research in various species, including dogs, suggests that interventions may be developed which can alter the underlying aging process and delay or prevent some age-associated conditions, including OA. One study in Labrador retrievers found that alterations in metabolism and body composition in dogs exposed to lifelong caloric restriction were associated with delayed onset of radiographic and clinical joint disorders.7,8 Novel approaches involving diet, exercise, and pharmaceuticals may be developed to capture these benefits in more targeted and pragmatic interventions.
The current study also supported the importance of body weight as a risk factor for OA. Higher body weight was significantly associated with increased risk, as was an increase in weight after full growth, which likely represented development of overweight or obesity. Larger dogs were at greater risk overall, and they were more significantly impacted by increased weight after maturity.
Body condition score (BCS) was also associated with an increased risk of OA diagnosis, but the magnitude of this effect was small. This is likely not an indication that overweight and obesity are not important risk factors but rather that there are significant limitations of BCS as a measure of this variable. In this study, over 80% of dogs were categorized as BCS=5 (normal weight) or BCS=7 (overweight). This limited range of scores reduced the sensitivity of BCS as a measurement tool.
BCS was a stronger predictor of OA diagnosis in smaller dogs, and there was also a wider range of scores assigned in these dogs, suggesting it may be easier for veterinarians to distinguish gradations of body condition in smaller patients. Developing more object and consistent alternatives to BCS as measures of overweight and obesity might make it easier to disentangle the relationships between body weight, body size, body condition, and OA risk.
Obviously, body condition is an important modifiable risk factor for clinicians to target. Overweight and obesity are risk factors for all-cause mortality, neoplasia, and other health conditions as well as for OA, so supporting clients in achieving a lean body condition in their dogs is a critical effort for reducing the prevalence of these conditions and improving companion dog welfare.9,10
Neuter status has been consistently identified as a risk for OA, with intact individuals at lower risk than neutered dogs.3 There is also evidence that earlier neutering is associated with an increased risk of OA and conditions predisposing to OA, though this effect is sometimes only significant in larger dogs. Consistent with this existing literature, the current study found neutered individuals were at higher risk of OA in large and medium dogs and in some, but not most, small breeds. Younger age at neutering was also associated with OA risk, though this effect diminished rapidly after 2 years of age. This relationship was found in dogs of all sizes, so the increased risk associated with earlier neutering did not appear to impact larger dogs more than smaller breeds.
Neutering is another potentially modifiable risk factor for OA. Owners can choose whether or not to neuter their dogs and at what age to perform the procedure. The existing evidence is consistent that delaying or forgoing neutering likely reduces the risk of OA later in life. However, the exact relationship between neuter status and OA is not clear.
Neutering does promote overweight and obesity, and it has been associated with increased risk of conditions that predispose to OA, such as cranial cruciate ligament rupture. In this study, the effect of body weight gain after maturity on OA risk was not different between intact and neutered dogs, so it did not appear that obesity was the main factor raising OA risk in neutered dogs. One previous study also suggested that obesity mediated only a small proportion of the increased OA risk associated with neutering.11 There remains, however, significant uncertainty about the exact nature of the relationship between neuter status, age at neutering, and OA.
Based on the existing evidence, it is reasonable to consider delaying neutering until full skeletal maturity or perhaps up to two years of age, especially in larger dogs. However, discussions about this between vets and clients must include the larger context of all the known risks and benefits of neutering.12,13 The net effect of neutering on the health and wellbeing of an individual dog is difficult to predict and will involve the influence of many other variables. Increased OA risk is only one consequence of neutering, and neuter status is only one of many factors associated with the development of OA. As always, clinical decisions should consider the complete and nuanced relationship between risks and benefits in the context of each individual patient.
Sex has been inconsistently reported as a risk factor for OA in previous research. In this study, males were at statistically lower risk than females, but the effect was small and likely not clinically meaningful. There were also breed differences in OA risk, and these were generally consistent with the pattern of larger breeds being at greater risk.
Breed is a complex risk factor involving differences in genetic makeup, body size and conformation, and potentially also lifestyle variables influenced by owners, such as feeding practices and the type and intensity of activity. Because of the enormous phenotypic variability among dogs, evaluation of the role of body weight in OA risk can easily confound the effects of body size and breed with those of overweight and other breed differences. This study confirmed the previously reported influence of body size on OA risk. Beyond that, other relevant breed variables were not examined.
Sex is, of course, not a modifiable OA risk factor in dogs. Breed may be modifiable on a population level to the extent that genetic risk factors and conformation can be impacted by selective breeding. However, from a clinical perspective, these factors are fixed and can only be factored into the overall assessment of risk for individual patients, not targeted to reduce risk.
There are many other risk factors for OA that were not evaluated in this study. Month of birth, level and type of activity during development, diet, and factors associated with the individual patient’s medical history, such as trauma and infectious diseases, are all potentially relevant to overall OA risk.3 The findings of this study, however, reinforce some of the most significant factors that clinicians can use to assess and modify OA risk in specific patients.
Maintaining a healthy body weight will certainly benefit all dogs. Delaying neutering may reduce the development of OA, particularly in larger dogs and those with other factors that put them at increased risk. In dogs that are at high risk due to size, breed, body condition, or personal history, more aggressive surveillance and treatment may be beneficial. As with all significant health conditions, OA risk must be evaluated and managed within the overall context of the specific case and with awareness of the nuances and uncertainties of the available evidence.
TAKE-HOME POINTS
REFERENCES
1. O’Neill DG, James H, Brodbelt DC, Church DB, Pegram C. Prevalence of commonly diagnosed disorders in UK dogs under primary veterinary care: results and applications. BMC Vet Res. 2021;17(1):1-14. doi:10.1186/S12917-021-02775-3/TABLES/2
2. Robinson NJ, Dean RS, Cobb M, Brennan ML. Investigating common clinical presentations in first opinion small animal consultations using direct observation. Vet Rec. 2015;176(18):463-463. doi:10.1136/VR.102751
3. Anderson KL, O’Neill DG, Brodbelt DC, et al. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci Rep 2018 81. 2018;8(1):1-12. doi:10.1038/s41598-018-23940-z
4. Johnston SA. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet Clin North Am Small Anim Pract. 1997;27(4):699-723. doi:10.1016/S0195-5616(97)50076-3
5. Millis D, Tichenor MG, Hecht S, Hunt T. Prevalence of Osteoarthritis in Dogs Undergoing Routine Dental Prophylaxis. In: World Small Animal Veterinary Association World Congress Proceedings. ; 2014.
6. Graves JL, McKenzie BA, Koch Z, Naka A, Spofford N, Morrison J. Body weight, gonadectomy, and other risk factors for diagnosis of osteoarthritis in companion dogs. Published online August 3, 2023:2023.08.03.550998. doi:10.1101/2023.08.03.550998
7. Huck JL, Biery DN, Lawler DF, et al. A Longitudinal Study of the Influence of Lifetime Food Restriction on Development of Osteoarthritis in the Canine Elbow. Vet Surg. 2009;38(2):192-198. doi:10.1111/J.1532-950X.2008.00487.X
8. Kealy RD, Lawler DF, Ballam JM, et al. Five-year longitudinal study on limited food consumption and development of osteoarthritis in coxofemoral joints of dogs. J Am Vet Med Assoc. 1997;210(2):222-225.
9. Marchi PH, Vendramini THA, Perini MP, et al. Obesity, inflammation, and cancer in dogs: Review and perspectives. Front Vet Sci. 2022;9:1004122. doi:10.3389/fvets.2022.1004122
10. Salt C, Morris PJ, Wilson D, Lund EM, German AJ. Association between life span and body condition in neutered client-owned dogs. J Vet Intern Med. 2019;33(1):89-99. doi:10.1111/jvim.15367
11. Simpson M, Albright S, Wolfe B, et al. Age at gonadectomy and risk of overweight/obesity and orthopedic injury in a cohort of Golden Retrievers. PLOS ONE. 2019;14(7):e0209131. doi:10.1371/journal.pone.0209131
12. McKenzie B. Evaluating the benefits and risks of neutering dogs and cats. CABI Rev. 2010;2010:1-18. doi:10.1079/PAVSNNR20105045
13. Hart BL, Hart LA, Thigpen AP, Willits NH. Assisting Decision-Making on Age of Neutering for 35 Breeds of Dogs: Associated Joint Disorders, Cancers, and Urinary Incontinence. Front Vet Sci. 2020;7:388-388. doi:10.3389/FVETS.2020.00388/BIBTEX
Obviously, the whole purpose of the SkeptVet is to combat misinformation and to promote evidence-based pet health. I first used the term Age of Endarkenment in a post for the much more influential Science-Based Medicine blog. Then, I was focused on the relatively narrow issue of the AVMA being unwilling to acknowledge the clearly evidence fact that homeopathy is useless pseudoscience and that vets shouldn’t offer it to clients (you can refresh yourself on the whole sorry saga in these posts).
When I was honored with the VIN Veritas Award, I was invited to give a rounds presentation on the Veterinary Information Network (unfortunately, you’ll need to be a member to view this). I used the opportunity to expand on the underlying cultural currents that have led to an apparent explosion in misinformation and mistrust of science, and to compare these to the principle soft the Age of Enlightenment, which underlie the achievements and progress science has brought to many fields, including healthcare.
I have since given a shortened version of this talk at the Western Veterinary Conference, and I will be giving this again at PacVet in San Francisco next month. Here is the slide deck and a summary of the talk.
WHAT IS THE DISEASE?
Mistrust of science and science-based medicine, as well as misinformation, pseudoscience, and false beliefs around medical topics have been around as long as science as a method for understanding nature has been around. We are currently in a moment when these problems are growing and spreading, and this threatens the health and wellbeing of humans and animals.
Misinformation is, by definition, information that is incorrect, not true. This may be a claim that is completely incorrect – for example, the assertion that vaccines are the cause of autism in children – or it may be something that is partially correct or even wholly correct, but in some way is misleading. The notion that vaccines have side effects is perfectly correct, for example. The notion that those side effects are far greater than their benefits is not correct. Misinformation can have elements of truth in it.
How do we know what is true or not true in terms of medical information? Ideally, we base that decision on the best current scientific evidence. Science is the best method that we have for understanding what’s true about nature and what’s not. Unfortunately, we can’t all be experts in every area of science or even every area of medicine. We do, to some extent, have to rely on the consensus of people who are experts in that area. This is probably the most problematic issue because people are often skeptical of experts, and we don’t like to be told what to think. We like to have our intellectual independence, which is a good virtue but gets us into trouble when we think the 20 minutes on the internet makes us an expert in any and all topics.
To deal with misinformation, we have to accept the fundamental premise that there are things that are true and things that are false, that science is probably the best way to distinguish those, and that sometimes we have to rely on the consensus of experts in a field to tell us what the truth is when we aren’t able to necessarily make that judgment ourselves.
HOW BAD IS IT?
The good news is that most people actually do trust science. If you survey people around the world, there are pretty high levels of trust in the things that scientists say about the natural world. That is still the majority of people almost everywhere.
People in particular trust medical scientists, especially doctors, and nurses. There are high levels of confidence in what medical people say about health and disease. Even though there is sometimes a worrisome degree of mistrust, we shouldn’t forget that the majority still think that scientists and medical scientists know what they’re doing and are on their side. This applies to veterinarians and veterinary technicians and nurses as well.
Unfortunately, mistrust and misunderstanding of science is still quite common. Here are some survey findings that should be very disturbing:
WHAT IS THE CAUSE?
Idols of the Tribe & the Cave- Psychological Causes
These are errors we make either because of how our brains function in general as a species, or particular sources of error that we have as individuals based on our personal experiences and inclinations.
Cognitive biases are systematic pattern of thinking arising from intrinsic features of our cognitive mechanisms. Research in psychology has uncovered an enormous number of cognitive biases that all human beings are prone to. These errors arise from faults in our memory, from quirks in how we direct our attention, and from the influence of our beliefs, desires, and expectations on what we observe. Many of these errors have direct relevance to clinical decision-making, and much of the methodology of science and evidence-based medicine (EBM) is designed specifically to correct for them.3
Logical fallacies are another psychological error source. These are arguments that are wrong, they’re invalid, and they don’t work – but they feel intuitively right and so are very hard to overcome. One example is the “false cause” fallacy. This states that if one event precedes another, the first event likely caused the second. The case of vaccines and autism is a paradigmatic example. Because children get vaccinated around the same time that autism symptoms emerge, there is a correlation in time. Because of the powerful force of this fallacy, it is very difficult to convince people this relationship isn’t causal despite strong evidence against it.
Idols of the Theater- Sociocultural Causes
Our beliefs about scientific topics are part of a larger understanding of the world we inhabit, and these beliefs are influenced by others. There is a strong psychological and social drive to keep all of these beliefs as consistent and coherent as possible and not to threaten our sense of belonging in social groups by challenging beliefs associated with membership. Political affiliation and religious beliefs are the predominant sociocultural influences that alter our views on scientific topis regardless of the objective evidence. These influences can often be quite arbitrary, and change over time.
A powerful recent example is the shift in views of science over the last forty years. In the 1970s, people with politically conservative affiliations tended to be strongly pro-science, associating science and technology with economic growth and military power. Those further to the left politically tended to be suspicious of science, as they were suspicious of industry and the military, and favorably inclined to alternative medicine and related ideas. This relationship has shifted dramatically, and today those professing a conservative political view are more likely to doubt science and scientific experts whereas slogans such as “Trust the Science” have become more common on the political Left. It is not science or scientific evidence which has changed, but the relationship between science and political identity.
Idols of the Marketplace- The Information Ecosystem
While it is by no means the only factor, there is no question that the internet plays a role in the growth of mistrust and misinformation about science. Social media, in particular, is driven by engagement. The algorithms, which prioritize some content and deprioritize other content, particularly amplify the extremes and controversy. Inflammatory content generates more active engagement, positive or negative, than more neutral or nuanced, fact-based content. Because of this, misinformation spreads faster and reaches more people than facts.
The internet also facilitates the creation of echo chambers. We now have the option of selecting our information sources at a very granular level so that we need not ever be bothered by information that contradicts our views if we don’t want to. This makes it very easy for us to become convinced that our beliefs, however mistaken or contrary to the evidence, are widely shared because the only people we interact with are people who think the same way. This amplifies misinformation, increases confidence in false beliefs, and locks people away from information that might challenge them and cause them to rethink.
WHAT IS THE HARM?
The World Health Organization has a whole set of resources to combat what it calls the “Infodemic-” the flood of information available to the public during a public health crisis. The U.S. Surgeon General also issued a report in 2021 address the critical importance of combatting misinformation and mistrust about public health.
The COVID pandemic was a strong example of an infodemic. Information was so voluminous that it overwhelmed people, and some began to tune out entirely, thereby missing important messages from reliable sources. There was also a lot of misinformation, and a lot of contradiction, which is confusing. All of that led to poor decision-making on the part of individuals and groups.
An analysis from the Brown School of Public Health estimated as many as 300, 000 adults in the United States died of COVID who would not have died had everyone eligible for the vaccine got it when it was available. The study also found that those individuals exposed to misinformation about vaccines were significantly less likely to be vaccinated, leading to an increased morbidity and mortality risk.
Mistrust and myths about science also harm those in science-based professions. Healthcare providers and public health officials have been subjected to historic levels of abuse, threats, and even violence from people misled into believing that the healthcare professions are a threat to them. This has exacerbated the flight from these fields and the resulting scarcity of healthcare services and public health expertise.
WHAT IS THE CURE?
There is no cure. Many of the roots of these problems are intrinsic to human cognition and social behavior, and those causes will almost certainly stay with us. However, there are treatments, measures we can take to build and maintain public trust in science and mitigate the harm misinformation does. These are the steps I recommend for veterinarians to combat this problem:
Veterinarians have extensive knowledge of science and medicine. Building and maintaining this knowledge base through conscientious, evidence-based medicine practices and then sharing this with our colleagues and clients is a natural and critical part of our role. Information challenging misinformation is most potent when it comes from trusted sources, and we are among the most trusted professions.
Understanding the causes of mistrust and false beliefs is necessary to combat them. If we write off people who distrust science or question our recommendations as stupid or hopeless, we cede the field to those who mislead them. Understanding how people find and maintain anti-science beliefs helps us immunize and treat them.
Likewise, understanding what makes for effective science communication gives our input more impact. There are many resources available to help us learn effective science communication skills, with clients and as members of our personal and professional communities.
Finally, we can have no impact if we don’t participate in the conversation. Be an advocate for your patients and your clients, so that they aren’t misled into things that are harmful. Be and advocate for your colleagues, and support them when they’re attacked and abused for saying things that are appropriate and truthful. Be and advocate for your community; participate in conversations about scientific issues in your community because you have a voice, and it’s an important one, and it can make a difference.
KEY “TAKE HOME” POINTS
REFERENCES
I recently reported on the results of a clinical trial conducted at North Carolin State University on the purported “anti-aging” Leap Years. The study provided no convincing evidence of a beneficial effect, and despite a single statistically significant finding at one time point, the data looked about as clearly negative as a study measuring multiple outcomes like this can.
Despite this, the company and its most prominent figure, Dr. David Sinclair, promoted it heavily as a major advance in canine geroscience. The pushback for these excessive and unsupported claims was surprisingly strong, and Dr. Sinclair had to resign the presidency of a major aging research organization he belongs to. That said. the product is still for sale and the company still makes many unproven claims for it, including some specifically based on this paper.
The original manuscript was released as a preprint, which is not peer reviewed, so when such studies are eventually published (if they are), sometimes the manuscript can change based on reviewers comments. The published version of this paper has now appeared, and there are pretty minimal changes.
As an example, here are the titles and abstracts from the two versions, with the differences bolded-
Preprint-
Randomized, Controlled Clinical Trial Demonstrates Improved Cognitive Function in Senior Dogs Supplemented with a Senolytic and NAD+ Precursor Combination
Peer-reviewed-
A randomized, controlled clinical trial demonstrates improved owner-assessed cognitive function in senior dogs receiving a senolytic and NAD+ precursor combination
Preprint-
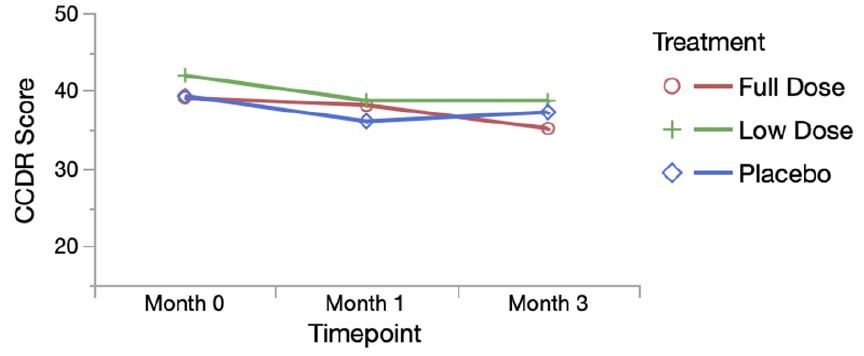
There was a significant difference in CCDR score across treatment groups from baseline to the primary endpoint (p=0.02) with the largest decrease in the full dose group. There were no significant differences between groups in changes in measured activity. However, the proportion of dogs that improved in frailty and owner-reported activity levels and happiness was higher in the full dose group than other groups. Adverse events occurred equally across groups. All groups showed improvement in cognition, frailty, and activity suggesting placebo effect and benefits of trial participation. We conclude that LY-D6/2 significantly improves owner-assessed cognitive function and may have broader effects on frailty, activity and happiness as reported by owners.
Peer-reviewed-
There was a significant difference in CCDR score across treatment groups from baseline to the primary endpoint (p = 0.02) with the largest decrease in the full dose group. No difference was detected between groups using in house cognitive testing. There were no significant differences between groups in changes in measured activity. The proportion of dogs that improved in frailty and owner-reported activity levels and happiness was higher in the full dose group than other groups, however this difference was not significant. Adverse events occurred equally across groups. All groups showed improvement in cognition, frailty, and activity suggesting placebo effect and benefits of trial participation. We conclude that LY-D6/2 improves owner-assessed cognitive function over a 3-month period and may have broader, but more subtle effects on frailty, activity and happiness as reported by owners.
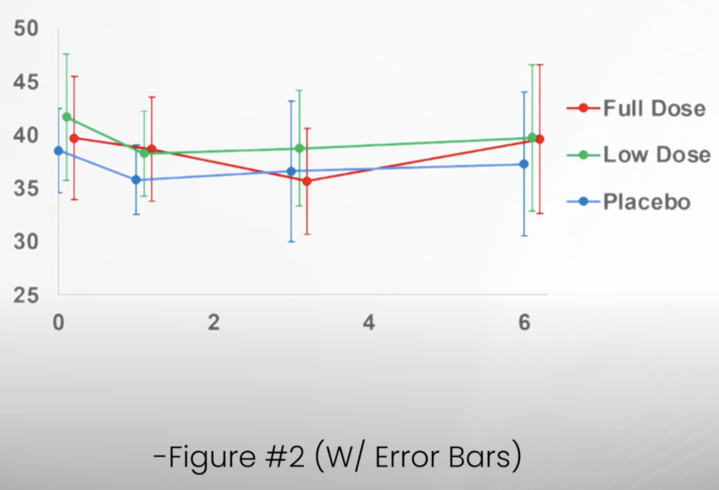
The intention of these small changes seems to be to walk back the level of confidence given for some of the findings and their significance. Similar changes appear in the discussion and elsewhere in the paper, but they don’t fundamentally change the reporting of results. I was a bit surprised that the reviewers did not recommend error bars be added to figure or that these be extended to include the 6-month evaluation. Both of these improvements to the figures emphasize the lack of a consistent and coherent pattern of response that would support meaningful benefits to the subjects.
Dr. Matt Kaeberline, an aging biology expert and strong critic of Dr. Sinclair, has prepared an amended figure based on the data the authors made available. The comparison between that figure and the one in the ;published article emphasizes how easy it is to present the data in one way and see the appearance if a pattern which is much less evident when the same data are presented in a different way.
Figure from paper-
Fire with error bars and 6-month data-
Bottom Line
The published paper is very similar to the preprint, which is not surprising since the original study was well-designed and conducted. The best case that can be made is that the supplement might have som effect on cognitive function in older dogs, but the existing evidence is underwhelming and more negative than positive overall. This might be enough to support further testing, but it certainly is not strong or sufficient to justify ongoing use, much less exuberant marketing of the product as an “anti-aging” treatment.
One of my most popular, and controversial, topics to talk about at continuing education meetings is the evidence for abandoning practices that are deeply entrenched in routine veterinary practice. Vets are pretty good at adopting new things when evidence shows these might be worthwhile, often even when the evidence isn’t very good. But giving up things we are used to doing is much harder, even when the evidence is strong. Here are a few things vets might want to think about changing, and you might want to question if they are offered to you.
CHOOSING WISELY: THINGS TO STOP DOING IN YOUR PRACTICE (MAYBE)
THE JOY AND THE PAIN OF EVIDENCE-BASED MEDICINE
Veterinary medicine is a science-based profession. The philosophy and principles of science, and the data generated by scientific research, guide our clinical decision-making. The joyful side of this is that we get to watch new tests and treatments emerge during our career, and previously hopeless problems become treatable. The scourge of parvoviral enteritis has been dramatically diminished thanks to the development of a vaccine. The “incurable” malady of feline infectious peritonitis now seems to be beaten, at least in many cases, by new drugs. The first wave of monoclonal antibody therapies is just arriving, offering more treatment options for diverse problems such as osteoarthritis and atopic dermatitis.
The painful side of an evidence-based approach to practice is that we are often wrong. Not only does early and incomplete science sometimes lead us to the wrong conclusions, but as individuals we make even less reliable judgements based on personal experience and anecdote. What is worse, we develop strong emotional and ideological commitments to these judgements. Giving up a therapeutic practice that we believe in, that we were taught as youngsters and that we have since taught to others, that we “have seen work” in our own hands, is deeply unsettling. We don’t like to be wrong, and we don’t like things that challenge our understanding of the world, because that damages our self-image and makes the world seem less predictable and controllable.1,2
But the welfare of our patients is more important than our ego and our sense of security, and we have an obligation as practitioners of a science-based art to follow the evidence where it leads. This will inevitably mean abandoning beliefs and practices that are dear to us repeatedly throughout our careers. So, let’s square our shoulders, raise our chins, and rip off a few of those band aids today!
WHAT IS EVIDENCE?
Ok, before we do that, we should take a minute to consider what “evidence” is and how we decide when it is good enough to justify changing our practices.3 You’ve all seen some image like Fig. 1 before. The details are less important than the general point—not all evidence is equally reliable. The bottom of the pyramid contains the most available and accessible evidence– our experiences and opinions and those of others. The top of the pyramid is the smallest because it represents the evidence that’s the hardest to get– consistent findings across multiple, replicated, high-quality controlled research studies. So, we have lots of the least trustworthy evidence and only a little of the best stuff.
Figure 1. Types of evidence categorized by quality and risk of bias.
But the problem is even worse than that! The stuff at the bottom is by far the most psychologically compelling. Our brains are built to understand and trust our own experiences and the stories other people tell us about theirs. Data and numbers from research studies are a lot less satisfying and convincing.4 So we are most confident in the least reliable evidence and least likely to be moved by the evidence that history has shown us, again and again, is most likely to be correct. Bummer!
Science is not, of course, a perfect process. It is simply something humans have developed over a long period of trial and error to compensate for our inherent limitations. Scientific studies can be wrong and misleading, just like anecdotal evidence. Not every study is well-designed, properly conducted and analyzed, and not every study applies to all patient populations. There are a million reasons why scientific research evidence might lead us to the wrong conclusion. But there are ten million reasons why experience and anecdotes will mislead us!
The best we can do is recognize the problem and make an honest effort to remind ourselves often that what feels true may not be if it is based on uncontrolled experience and anecdote. If you find yourself saying, “But, I’ve seen it work!” take a deep breath and try to remind yourself that the proponents of blood-letting, magic healing rituals, and any therapy you believe is useless say exactly the same thing.
USES OF ANTIBIOTICS TO RECONSIDER
Treatment of Acute Diarrhea
The use of metronidazole as a treatment for acute diarrhea in dogs is a deeply entrenched practice that goes back decades. Various rationales have been proposed to support it, from treatment of potential bacterial causes, such as Clostridium, to anti-inflammatory mechanisms. Few general practice vets have not used this drug for this purpose, and there is abundant anecdotal evidence suggesting it is beneficial.
Unfortunately, there is also a growing body of research evidence showing little to no clinical benefit for most patients and some potential undesirable effects.5 At best, it might shorten the course of diarrhea by about a day. At worst, it can make symptoms worse, disrupt the microbiome in potentially harmful ways, and contribute to antibiotic resistance.
Given that the vast majority of dogs with idiopathic acute diarrhea will get better with time, these risks are hard to justify. Most humans don’t seek medical are or prescription drugs for mild, short-term diarrhea symptoms. The pressure to treat this condition in dogs has more to do with the inconvenience and anxiety it causes owners than the wellbeing of our patients. Although the risks seem small, it is difficult to justify them for a treatment that has mostly psychological benefits for vets and clients rather than medical benefits for patients.
It would be nice to have a clear alternative to offer here, but the reality is no research yet supports any specific treatment for acute, self-limiting diarrhea that is clearly effective and has negligible risks. Probiotics have not entirely lived up to their promise.6 Some evidence supports dietary change and fiber supplementation,7,8 but again most cases are self-limiting and likely to get better without any specific treatment.
Treatment of Upper Respiratory Infections
Like acute diarrhea, acute upper respiratory infections (URI) are often self-limiting in dogs and cats, and many have viral etiologies which will not respond to antibiotic treatment. There is limited controlled research comparing antibiotic use to alternatives, such as supportive care alone. Based on the clinical research that we do have, and also the basic science studies providing background on the causes and outcomes of feline and canine upper respiratory disease, expert consensus guidelines generally recommend limiting antibiotic use to cases with significant systemic symptoms (e.g. fever, lethargy) and evidence of bacterial involvement (e.g. mucopurulent discharge), or cases with chronic disease.9
Unfortunately, the lack of evidence for benefits from antibiotic treatment in most cases, and the potential for adverse effects and microbial resistance, vets still seem to often prescribe antibiotics unnecessarily for canine and feline respiratory infections.10,11 There is some indication, however, that awareness of, and adherence to, antibiotic use guidelines may be improving.12
Treatment of Urinary Tract Infections
Unlike upper respiratory disease, urinary tract symptoms often are caused by bacterial infection in dogs, and to a lesser extent in cats. These bacterial urinary tract infections (UTI) do sometimes require antibiotic treatment, though common practice in treating UTIs still often does not match current evidence-based guidelines.13
For one thing, just having bacteria in the urine does not a UTI make. Asymptomatic bacteriuria seems to be more common than previously recognized, occurring in from 1% to 13% of healthy dogs and cats, and at much higher rates in animals with immunosuppressive conditions or medications and other risk factors.13 In the absence of clinical symptoms, treatment with antibiotics does not permanently eliminate bacteriuria and appears to have no benefits for the patient. While evidence is somewhat limited in dogs and cats, it is clear that in humans prescribing antibiotics for subclinical bacteriuria raises the risk of adverse drug effects and antibiotic resistance without improving short-term or long-term outcomes for patients. This is true even if there is pyuria!13
Recommended treatment of symptomatic UTI confirmed by urine culture is also different from what many of us were taught long ago. Some of these cases may not require antibiotics at all. Humans with uncomplicated UTI are often treated symptomatically with NSAIDs, and the UTI often resolves by itself. This may be appropriate for veterinary patients too, though we do not yet have studies showing this.
Similarly, the recommended duration of treatment is 3-5 days, which is far shorter than the 7-10 day course many of us still prescribe. Even pyelonephritis is typically treated in humans with 7-10 days of antibiotics, and in the absence of better evidence experts currently recommend 10-14 days of treatment in dogs and cats, rather than the 4-6 weeks previously advised.13
One of the most common inappropriate uses of antibiotics for urinary tract signs is for young cats. In cats under about ten years of age hematuria, pollakiuria, and other symptoms of cystitis are far less likely to be caused by UTI (2%-19%) than in cats over 10 years of age (40-45%).14 Feline Interstitial Cystitis (FIC) is the most common cause of lower urinary tract symptoms, and of course antibiotics are not a useful or appropriate treatment for cats with FIC.15
Much of the antibiotic prescribing for urinary tract symptoms, as for gastrointestinal and respiratory symptoms, is driven by psychological factors: owner anxiety and demands for treatment, veterinarian anxiety about negative consequences to undertreating, and simple commission bias (the need to DO SOMETHING rather than wait for self-limiting problems to resolve on their own).4 Given that antibiotics are safe but not entirely benign, and that we are losing some effective treatments for serious infections to antimicrobial resistance, we should do what we can to resist the siren-song of antibiotic prescription above and beyond what is likely needed.
Perioperative Antibiotics
Speaking of anxiety, the prospect of a post-operative infection appears to haunt the nightmares of veterinarians, based on the rate at which many of us prescribe antibiotics for our surgical patients.
Available evidence suggests that surgical site infections are uncommon for most clean or clean-contaminated procedures (< 5%), and that giving antibiotics before or after surgery do nothing to prevent these. Even in cases with specific risk factors for infection, the most we should likely do is provide appropriate antibiotic coverage from 30-60 minutes before the procedure until 6 to 24 hours afterwards.16 There is no convincing rationale or evidence, from human or veterinary medicine, to support more extensive antibiotic use to prevent surgical site infections.
Veterinary dentistry is a special case of perioperative antibiotic use.17 Once again, extensive evidence in human medicine shows that bacteremia occurs with chewing, brushing, or flossing just as it does with dental procedures, and that antibiotics are not useful for routine prophylactic procedures. Only people at high risk of infective complications (those with implant foreign bodies, a history of certain cardiac diseases, or those who are immunosuppressed) are likely to benefit from antibiotics when having dental work.
There is a lot less data in veterinary patients, but the expert consensus is that antibiotics are rarely necessary for dentistry patients and are not justified except in those at high risk. Unfortunately, it isn’t clear who those patients are. Infective endocarditis is often suggested as a potential risk from dental procedures, but this appears to be extremely rare in dogs and cats. Overall, antibiotics are very unlikely to benefit the vast majority of veterinary dentistry patients, and their risks likely outweigh their benefits.17
Despite this, it appears that antibiotics are very commonly used in veterinary dentistry. In dogs and cats with established periodontal disease or requiring extractions (which describes most dentistry patients), antibiotic use appears to be very common, though use
varies widely between different veterinary facilities.18 More evidence and clearer guidelines would likely help reduce the overuse of antibiotics in veterinary dentistry.
USES OF ANALGESICS TO RECONSIDER
Tramadol
If ever there was a cautionary tale veterinarians should heed about an inappropriately used analgesic, it is the story of tramadol. A cheap and widely used opioid and serotonin agonist in human medicine,19 tramadol became a popular pain therapy in veterinary species despite some pretty significant warning signs that our enthusiasm for the drug was premature. Because it is a pro-drug that has to be converted to an active metabolite to have an effect, it should have been a red flag that this conversion is much less efficient in dogs than in humans.20,21
Nevertheless, it became popular for post-operative pain and for dogs with osteoarthritis. The use of tramadol was likely driven by anxiety about NSAIDs, which are the most extensive studies and most clearly effective oral analgesic for dogs and cats. Despite lots of data and a strong overall safety record,22 vets and pet owners seem especially worried about the potential risks of NSIADs and eager to adopt any alternative portrayed as safer, even when the data are scant.
Eventually, clinical studies accumulated showing that tramadol has marginal benefits over placebo for dogs.23 Caregiver placebo effect likely accounted for most of the appearance of benefits. And while tramadol has more of an analgesic effect in cats, due to more efficient conversion to the active metabolite, it also has pretty significant adverse effects in this species.24 Sadly, many dogs undoubtedly experienced inadequately treated acute and chronic pain, and some still do, because of our eagerness to accept a purported NSAID alternative and our willingness to believe anecdotal evidence for what we were hoping and expecting to see.
Gabapentin
The authors of the 2022 American Animal Hospital Association pain management guidelines for dogs and cats, at least, are trying to prevent history from repeating itself. They state, “Gabapentin has become the new tramadol, with widespread usage [despite] virtually no supporting data.”25
While there is good evidence in humans to support use of gabapentin for seizures and for some specific types of neuropathic pain (post-herpetic neuralgia and diabetic neuropathy), even most analgesic use in humans is without much supporting research evidence.26 In dogs and cats, there is limited evidence to support using gabapentin to reduce behavioral signs of stress, although it isn’t entirely clear if it reduces anxiety or is primarily a sedative. However, there is little reason to expect it to be of great benefit for acute or chronic pain. While more research needs to be done, it seems likely we have failed to learn our lesson from tramadol and are continuing down a similar road with this drug.
Mixing Lidocaine and Bupivicaine for Local Blocks
This is one of those ideas that sounds brilliant but is actually totally wrong!27 Some clinicians mix lidocaine and bupivacaine together with the idea that they will get “the best of both worlds–” the more rapid onset of lidocaine with the longer duration of bupivacaine. What actually happens is that you get the worst of both worlds!
The dilution of each drug leads to a lower concentration gradient, meaning less of both end up getting into the nerves where they act to block pain. The difference in pH also means that mixing them likely slows the uptake of the lidocaine and might make the bupivacaine more likely to precipitate. Clinical studies in actual human and veterinary patients have also shown that this practice does not reduce the time to effect, but it does shorten the duration.
Steroids for Intervertebral Disk Disease (IVDD)
Acute medical management of IVDD mostly involves controlling pain and trying to prevent further injury or loss of function. Steroids have long been used for both analgesia and to protect from further deterioration of nerve tissue by reducing inflammation. Unfortunately, it has been difficult to produce research evidence that supports the real-world benefits of this approach. In humans, the use of oral steroids does not appear very beneficial, though it does have a higher incidence of adverse effects than other analgesics. The evidence in veterinary patient is sparse, but the most recent ACVIM consensus statement on the subject indicates that:28
The best we can say is that steroids are probably no better than NSAIDs, and it seems likely they may be worse.
MISCELLANEOUS TREATMENTS TO RECONSIDER
ACE Inhibitors for Pre-clinical Mitral Valve Disease
Diagnosis and staging of mitral valve disease (MMVD) before dogs are in congestive heart failure (CHF) is now pretty rewarding. The advent of pimobendane has given us an intervention that likely delays the onset of CHF and extend life significantly for dogs with this disease.29 It wasn’t always so.
Back in the Dark Ages that make up most of my career, we used to give these dogs ace inhibitors (ACE-I). There were some sound theoretical arguments to suggest this would slow the progression to CHF. Unfortunately, a few pesky scientists weren’t satisfied with theory, and they did some clinical studies in actual MMVD patients. They found that that we were most likely wasting our time.
As the most recent systematic review puts it, “Administration of angiotensin-converting enzyme inhibitors to dogs with preclinical myxomatous mitral valve disease…results in little to no difference in the risk of the development of congestive heart failure and may result in little to no difference in cardiovascular-related and all-cause mortality.”30
Good thing we’ve all stopped doing this, eh?
Glucosamine for Osteoarthritis (OA)
Probably the most popular (and profitable!) supplement in the history of veterinary medicine is glucosamine, alone or mixed with chondroitin or other agents. If everyone has used it forever, it has to work, right?!
Well, it turns out there is some controversy about that. Decades of research, hundreds of studies, are currently summarized in dozens of systematic reviews over the last ten years. For humans, these are often broken down into treatment for OA in specific joints, and there is sometimes analysis of glucosamine alone, in combination with other agents, or in different formulations and dosages. The result is muddled, and no clear, universal conclusions are possible.
About 60% of the reviews conclude there is some benefit to some type of glucosamine-containing product, and the other 40% conclude no meaningful benefit or insufficient evidence to tell. Not exactly a ringing endorsement for one of the most widely used supplements ever. The most recent systematic review for veterinary patients is ambivalent:31
“As we exposed in this review, glucosamine and chondroitin sulfate seems to provide chondroprotective effects and less inflammatory biochemical response in approximately half of the evaluations. However, these effects are inconsistent between the clinical and the preclinical studies… a possible caregiver placebo effect may explain some of the beneficial responses observed in clinical trials with dogs.”
The latest guideline from the American College of Rheumatology and the Arthritis Foundation, “recommends against glucosamine alone or with chondroitin because treatment does not improve knee and hip OA in studies without industry funding.”32 Ouch!
The American Academy of Orthopedic Surgeons say glucosamine “May be helpful in reducing pain and improving function…however, the research is inconsistent/limited.”33
There aren’t official guidelines from specialty groups in veterinary osteoarthritis management, but a recent proposed expert consensus statement highlighted the “lack of evidence” for efficacy needed to draw a firm conclusion.34 Another similar consensus statement indicated that three or four of nine contributing experts recommended offering some form of glucosamine for patients with OA depending on the specific circumstances.35
Despite being widely used for decades, it has proven impossible to clearly demonstrate that glucosamine in some form or combination has meaningful benefits for comfort and function in veterinary patients with OA. It is pretty well demonstrated to be safe, so there is unlikely to be any direct harm from using it. However, if it is ultimately not truly beneficial, what a horrendous waste of money it will have been for owners. And if it is used in place of clearly beneficial treatments (as it likely is, given how phobic people often are about NSAIDs), many OA patients could be suffering unnecessarily.
REFERENCES
1. Burton RA. On Being Certain: Believing You Are Right Even When You’re Not. 1st ed. St. Martin’s Press; 2008. Accessed April 3, 2024. http://catdir.loc.gov/catdir/enhancements/fy0829/2008001470-s.html
2. Gilovich T. How We Know What Isn’t so: The Fallibility of Human Reason in Everyday Life. 1. Free Press paperback ed. Free Press; 1993.
3. McKenzie B. Evidence-based veterinary medicine: What is it and why does it matter? Equine Vet Educ. 2014;26(9):451-452. doi:10.1111/eve.12216
4. McKenzie BA. Veterinary clinical decision-making: cognitive biases, external constraints, and strategies for improvement. J Am Vet Med Assoc. 2014;244(3):271-276. doi:10.2460/javma.244.3.271
5. Scahill K, Jessen LR, Prior C, et al. Efficacy of antimicrobial and nutraceutical treatment for canine acute diarrhoea: A systematic review and meta-analysis for European Network for Optimization of Antimicrobial Therapy (ENOVAT) guidelines. Vet J. 2024;303:106054. doi:10.1016/j.tvjl.2023.106054
6. Jensen AP, Bjørnvad CR. Clinical effect of probiotics in prevention or treatment of gastrointestinal disease in dogs: A systematic review. J Vet Intern Med. 2019;33(5):1849-1864. doi:10.1111/jvim.15554
7. Lappin MR, Zug A, Hovenga C, Gagne J, Cross E. Efficacy of feeding a diet containing a high concentration of mixed fiber sources for management of acute large bowel diarrhea in dogs in shelters. J Vet Intern Med. 2022;36(2):488-492. doi:10.1111/jvim.16360
8. Moreno AA, Parker VJ, Winston JA, Rudinsky AJ. Dietary fiber aids in the management of canine and feline gastrointestinal disease. J Am Vet Med Assoc. 2022;260(S3):S33-S45. doi:10.2460/javma.22.08.0351
9. Lappin MR, Blondeau J, Boothe D, et al. Antimicrobial use Guidelines for Treatment of Respiratory Tract Disease in Dogs and Cats: Antimicrobial Guidelines Working Group of the International Society for Companion Animal Infectious Diseases. J Vet Intern Med. 2017;31(2):279-294. doi:10.1111/jvim.14627
10. Bollig ER, Granick JL, Webb TL, Ward C, Beaudoin AL. A quarterly survey of antibiotic prescribing in small animal and equine practices—Minnesota and North Dakota, 2020. Zoonoses Public Health. 2022;69(7):864-874. doi:10.1111/zph.12979
11. Robbins SN, Goggs R, Lhermie G, Lalonde-Paul DF, Menard J. Antimicrobial Prescribing Practices in Small Animal Emergency and Critical Care. Front Vet Sci. 2020;7. doi:10.3389/fvets.2020.00110
12. Farrell S, Bagcigil AF, Chaintoutis SC, et al. A multinational survey of companion animal veterinary clinicians: How can antimicrobial stewardship guidelines be optimised for the target stakeholder? Vet J. 2024;303:106045. doi:10.1016/j.tvjl.2023.106045
13. Weese JS, Blondeau J, Boothe D, et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet J. 2019;247:8-25. doi:10.1016/j.tvjl.2019.02.008
14. Dorsch R, Teichmann-Knorrn S, Sjetne Lund H. Urinary tract infection and subclinical bacteriuria in cats: A clinical update. J Feline Med Surg. 2019;21(11):1023-1038. doi:10.1177/1098612X19880435
15. He C, Fan K, Hao Z, Tang N, Li G, Wang S. Prevalence, Risk Factors, Pathophysiology, Potential Biomarkers and Management of Feline Idiopathic Cystitis: An Update Review. Front Vet Sci. 2022;9:900847. doi:10.3389/fvets.2022.900847
16. Williams J. Antimicrobial prophylaxis: The why and how of antimicrobial prophylaxis. BSAVA Companion. 2018;2018(11):4-7. doi:10.22233/20412495.1118.4
17. Davis E. The Use of Antibiotics in Veterinary Dentistry. Today’s Veterinary Practice. Published April 14, 2023. Accessed April 3, 2024. https://todaysveterinarypractice.com/dentistry/antibiotics-in-veterinary-dentistry/
18. Weese JS, Battersby I, Morrison J, Spofford N, Soltero-Rivera M. Antimicrobial use practices in canine and feline dental procedures performed in primary care veterinary practices in the United States. PLOS ONE. 2023;18(12):e0295070. doi:10.1371/journal.pone.0295070
19. Subedi M, Bajaj S, Kumar MS, Yc M. An overview of tramadol and its usage in pain management and future perspective. Biomed Pharmacother Biomedecine Pharmacother. 2019;111:443-451. doi:10.1016/j.biopha.2018.12.085
20. Benitez ME, Roush JK, KuKanich B, McMurphy R. Pharmacokinetics of hydrocodone and tramadol administered for control of postoperative pain in dogs following tibial plateau leveling osteotomy. Am J Vet Res. 2015;76(9):763-770. doi:10.2460/ajvr.76.9.763
21. Schütter AF, Tünsmeyer J, Kästner SBR. Influence of tramadol on acute thermal and mechanical cutaneous nociception in dogs. Vet Anaesth Analg. 2017;44(2):309-316. doi:10.1016/j.vaa.2016.02.003
22. Monteiro-Steagall BP, Steagall PVM, Lascelles BDX. Systematic review of nonsteroidal anti-inflammatory drug-induced adverse effects in dogs. J Vet Intern Med. 2013;27(5):1011-1019. doi:10.1111/jvim.12127
23. Donati PA, Tarragona L, Franco JVA, et al. Efficacy of tramadol for postoperative pain management in dogs: systematic review and meta-analysis. Vet Anaesth Analg. 2021;48(3):283-296. doi:10.1016/j.vaa.2021.01.003
24. Guedes AGP, Meadows JM, Pypendop BH, Johnson EG. Evaluation of tramadol for treatment of osteoarthritis in geriatric cats. J Am Vet Med Assoc. 2018;252(5):565-571. doi:10.2460/javma.252.5.565
25. Gruen ME, Lascelles BDX, Colleran E, et al. 2022 AAHA Pain Management Guidelines for Dogs and Cats. J Am Anim Hosp Assoc. 2022;58(2):55-76. doi:10.5326/JAAHA-MS-7292
26. Chincholkar M. Gabapentinoids: pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br J Pain. 2020;14(2):104-114. doi:10.1177/2049463720912496
27. Hoffmeister E. Mixing local anesthetics – yay or nay? North American Veterinary Anesthesia Society. Published September 27, 2019. Accessed April 3, 2024. https://www.mynavas.org/post/mixing-local-anesthetics-yay-or-nay
28. Olby NJ, Moore SA, Brisson B, et al. ACVIM consensus statement on diagnosis and management of acute canine thoracolumbar intervertebral disc extrusion. J Vet Intern Med. 2022;36(5):1570-1596. doi:10.1111/jvim.16480
29. Boswood A, Häggström J, Gordon SG, et al. Effect of Pimobendan in Dogs with Preclinical Myxomatous Mitral Valve Disease and Cardiomegaly: The EPIC Study-A Randomized Clinical Trial. J Vet Intern Med. 2016;30(6):1765-1779. doi:10.1111/jvim.14586
30. Angiotensin?converting enzyme inhibitors in preclinical myxomatous mitral valve disease in dogs: systematic review and meta?analysis – Donati – 2022 – Journal of Small Animal Practice – Wiley Online Library. Accessed April 3, 2024. https://onlinelibrary.wiley.com/doi/10.1111/jsap.13461
31. Barbeau-Grégoire M, Otis C, Cournoyer A, Moreau M, Lussier B, Troncy E. A 2022 Systematic Review and Meta-Analysis of Enriched Therapeutic Diets and Nutraceuticals in Canine and Feline Osteoarthritis. Int J Mol Sci. 2022;23(18):10384. doi:10.3390/ijms231810384
32. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020;72(2):149-162. doi:10.1002/acr.24131
33. Brophy RH, Fillingham YA. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. JAAOS – J Am Acad Orthop Surg. 2022;30(9):e721. doi:10.5435/JAAOS-D-21-01233
34. Mosley C, Edwards T, Romano L, et al. Proposed Canadian Consensus Guidelines on Osteoarthritis Treatment Based on OA-COAST Stages 1–4. Front Vet Sci. 2022;9. doi:10.3389/fvets.2022.830098
35. Cachon T, Frykman O, Innes JF, et al. COAST Development Group’s international consensus guidelines for the treatment of canine osteoarthritis. Front Vet Sci. 2023;10:1137888. doi:10.3389/fvets.2023.1137888
I am preparing a number of conference presentations for this year, and one of the new ones is on the topic of Spectrum of Care. This is a concept I have been involved this for a while, since participating in a working group organized by a classmate of mine that culminated in a publication kicking off a discussion about balancing the needs of patients and the rising costs of veterinary care.
Below are some thoughts on the subject. If you want more, I will be talking about this (and lots of other topics) at the Pacific Veterinary Conference in San Francisco July 11-12, 2024.
WHAT IS SPECTRUM OF CARE?
The core of the Spectrum of Care (SOC) concept is recognizing that the treatment of each patient takes place in a unique context that includes the patient, the client, and the veterinarian.1–3 There is no single, universal “gold standard” that can be automatically applied to every patient. A key role for the practitioner is to identify the best approach for a specific case by integrating-
An articulated spectrum of care approach should provide support and guidance to practitioners and ensure consistent and explicit consideration of all relevant information by both vets and clients. Vets provide recommendations for managing the case, always relying on an evidence-based understanding of the needs of the patient and of the risks, benefits, and uncertainties associated with the available interventions. These recommendations are then adapted to the goals and capacity of the owner through an explicit shared decision-making process.
The impact of the approach ultimately chosen on the wellbeing of the patient is then regularly re-assessed, and the plan is adjusted as needed throughout the course of treatment. Such adjustment always involves clear discussion between the vet and the client about all relevant issues, including the apparent benefits and adverse effects on the patient, the caregiver burden, the practical and economic sustainability of treatment, and the ultimate goals as understood by the vet and the owner.
PATIENT FACTORS
The needs and quality of life of the patient are always the central focus of medical care. These should be assessed in as objective and evidence-based a way as possible. The use of clinical metrology instruments, such as pain scales and quality of life tools, can help veterinarians and owners gain a more accurate common understanding of how the patient is doing and what they need.4,5
While veterinary patients cannot intellectually understand their condition and consent to treatment, they do have feelings and reactions to their care that must be taken into account. If a patient has a strong fearful or aggressive reaction to treatment, or if assessment suggests that treatment itself is negatively affective quality of life, alternative approaches should be considered.
Because our goal is to maintain or to reestablish a state of comfortable living in which patients can experience the activities and interactions they enjoy, all possible efforts should be made to ascertain what a good quality of life is for a given patient and to align the veterinarian’s and the clients’ understanding of this to the greatest extent possible. This will help guide the choices both parties make among the spectrum of care options available.
CLIENT FACTORS
The factor vets think about most often when considering the impact of clients on the treatments we can use is money. There is no question that financial constraints very often limit which tests and treatments can be employed, and clinicians must be adept at finding ways to meet the patient’s needs and the clients’ goals while not undermining the financial viability of their own practices.
An explicit spectrum of care approach that supports early and open discussion of such issues can help avoid wasting limited resources on tests that will not change the treatment plan or outcome and on treatments that are not effective or sustainable for the client. The sooner and more openly we talk about the availability of multiple paths towards achieving an acceptable outcome for the patient, the more efficiently we will choose and begin following the right path.
Money is not, however, the only client variable that impacts the choice of treatment. There is a growing literature concerning caregiver burden, which includes the physical, social, and emotional impact of caring for a sick pet as well as the financial costs.6,7 There are even instruments for measuring caregiver burden. Expanded development and use of these could be very helpful for vets and clients in understanding the overall constraints on care options and in making more thoughtful and effective choices.
The personal beliefs and values of clients can also affect their care decisions. Clients who have misconceptions or misunderstandings about the safety and effectiveness of medical treatments may need to be educated to inform better choices. And clients who have strong ideological or spiritual beliefs related to healthcare may choose some options and eschew others to stay consistent with these beliefs, regardless of the scientific evidence.
This can be frustrating when the veterinarian has a very different understanding of the situation. Seeing a patient suffering because a client is opposed to potentially beneficial therapies or categorically rejects the option of euthanasia is just as distressing for vets as when a client cannot afford needed care for their pet.
We cannot always achieve what feels like an optimal outcome for our patients in these situations. However, an explicit SOC approach with an emphasis on open communication about such issues and about all the available options may lead to better outcomes for patients than the extremes of an absolutist “my way or the highway” approach or a complete surrender to whatever the client demands.
VETERINARIAN FACTORS
In order to offer a range of diagnostic and treatment options that will effectively address the patient’s needs, vets must be aware of the options available and the risks, benefits, and uncertainties for each. An accurate, evidence-based understanding of these is essential, because offering ineffective treatments or avoiding those that actually work can never benefit patients. Our role in the VCPR is to understand what the patient needs and how we can best achieve this from a science-based perspective that is not directly available to the owner. We then need to advocate for the patient and educate the owner to provide them with the perspective and information necessary for making appropriate care choices.
Just as the client has constraints on their capacity to utilize particular treatments, so vets are constrained by their own knowledge and skills and by the resources available to them. The optimal therapy for a given case may not be available if it involves drugs or equipment the vet doesn’t have, techniques they are not familiar with, or staffing resources that exceed what is available in their practice.
The majority of veterinary assessment and treatment occurs in primary care practices, and this is where the implementation of an SOC approach from the initial encounter is especially needed. Optimal management of a particular patient may be possible in general practice, or it may require some level of specialty consultation or referral.8 One key component of SOC is determining if and when referral may benefit patients and how to achieve the best possible outcome for them when this is not an option. Often, the utility of referral is determined not only by the medical needs of the patient but by the capabilities of the practitioner, the goals and capacity of the owner, and the nature of communication within the VCPR.
COMMUNICATION ABOUT SPECTRUM OF CARE
Veterinarians are essential for effective patient care because we have the medical knowledge to understand the needs of the patient and to offer treatments to meet these. This does not, of course, mean that the client factors already discussed are not critical. The client can often understand the needs of the patient from a perspective not accessible to the veterinarian, and aligning these two perspectives is a key goal of the shared decision-making process. Just as the client cannot make effective decisions without accurate information about their options, the veterinarian cannot offer the most useful guidance if they do not understand and incorporate the clients’ perspective and needs into their recommendations.
To support the best possible care choices, the owner should understand the risks, benefits, and costs of intervention and the expected outcome associated with different approach, as well as the degree of uncertainty involved. The vet should understand the owners’ beliefs, expectations, and limitations well enough to recognize whether recommended interventions can be successfully employed or not. If a particular approach is unlikely to be effective due to client or patient factors, then alternative approaches to obtain an acceptable outcome should be considered and discussed.
Veterinarians are already accustomed to the negotiation and compromises between what they may see as the medically optimal approach and what clients are able or willing to do. However, such compromises are often made in an idiosyncratic manner, according to the particular beliefs, experiences, and habits of the individual clinician. The focus is usually on the financial constraints imposed by the client and how this hampers the “gold standard” care that a vet would like to provide. This process is often framed as a veterinarian recommending the optimal medical treatment and the owner declining, with the vet ultimately agreeing, shamefully or with resignation, to a cheaper and less appropriate treatment approach.
This pejorative view mischaracterizes a necessary process and can leave both clinicians and owners feeling they have failed to provide good care for the patient. It also hampers thoughtful decision making and open communication, which could make the inevitable compromises more rational as well as less unpleasant for everyone.
The concept of a medical “gold standard” is mostly mythical and counterproductive.9 There are no universal treatment approaches that can be automatically applied to every possible case. Veterinary students are still trained predominantly by specialists in tertiary care facilities, often learning their craft from the least representative exemplars of the profession and seeing patients and interventions very different from those common in general practice. This creates a clash of expectations and perspectives that must be resolved once new graduates enter a primary care practice environment.8 One goal of the SOC approach is to legitimize the inevitable differences between these practice contexts and diminish the perception that tertiary care represents to optimal or most appropriate approach while primary care is a lesser, fallback alternative.
Vets need support in developing the communication skills necessary to build effective VCPRs in a diverse client population with varied beliefs, goals, values, and constraints on the care they can provide for their pets. The SOC approach is aimed specifically at providing this support and normalizing the process of developing a management plan within the unique context of each case without the stigma that is currently attached to this process. Official recognition of the value and legitimacy of a SOC approach can also protect veterinarians from the anxiety that they will be punished, legally, financially, or in reputation, for appropriately adapting the available treatment options to the circumstances of each case.
THE ROLE OF EVIDENCE-BASED MEDICINE IN SOC
The lack of a single universal approach which all vets should employ reflexively does not, of course, mean that general principles and guidelines are not useful. These can be extremely helpful to inform decision-making, especially in characterizing the needs of the patient and the risks and benefits of various possible interventions.
Options included within the spectrum of care choices must be effective, and this means not only acceptable to the client and the veterinarian but also with a demonstrably positive balance between risks and benefits. Scientific evidence and the processes of evidence-based medicine are the best tools to identify the patient needs and the strengths and weaknesses of various treatment options.10
The SOC approach also does not render all such options equally appropriate. There are still “wrong” choices, from both a scientific and an ethical perspective. Ineffective treatments, or those which do more harm than good, are no more acceptable within the SOC approach than treatments which the client cannot afford or which the patient cannot tolerate. The discretion of the clinician to adapt general guidelines to the context of a specific case is constrained by scientific truth as well as by the resources or choices of the client.
The development and greater use of evidence-based clinical practice guidelines and tools for assessing quality of life and caregiver burden can facilitate a beneficial SOC practice. More research is also needed to characterize the risks and benefits of alternative treatment approaches for specific conditions. For example, studies comparing patient outcomes between routinely recommended and alternative, less intensive and expensive, treatments for canine parvoviral enteritis and for urinary tract obstruction in cats illustrate how such information can inform discussions between vets and owners about the pros and cons of various treatment options.11,12
Less aggressive treatment may have lower chances of a desired outcome, and clients should know this. However, the chances of a good result are still likely to be greater than when euthanasia is chosen over care that the client cannot afford or the veterinarian cannot provide. And less intensive care may also have fewer adverse effects and a lower caregiver burden, which could provide for better quality of life for the pet even if, in some cases, the length of life might be shorter. Such tradeoffs are inevitable and already commonly made, but vets and clients cannot make them effectively and maximize the potential for acceptable patient outcomes without accurate information indicating the risks and benefits of various approaches.
CONCLUSIONS
Offering a spectrum of care options that meet patient needs and aligns with the goals and capacity of vets and clients is not a revolutionary new idea. Vets have been negotiating the best approach for the unique circumstances of each case forever.
However, SOC is also not the same old thing we have long been doing. The usual negotiations depend very much on the idiosyncratic beliefs, experiences, and personalities of individual veterinarians. What interventions we offer, how we anticipate or respond to constraints imposed by clients, and how we manage the critical communication within the VCPR all depend on ad hoc approaches we each develop individually.
An articulated SOC approach emphasizes an evidence-based understanding of the options available, explicit and open communication about what the patient needs and what the vet and the owner can provide, and shared decision-making for how best to achieve a good outcome for the patient within the inevitable constraints of circumstances. SOC brings structure and transparency to this process and removes the shame created by the myth of a universal “gold standard” for care and the indoctrination in school that tertiary care practices represent the ideal for which all vets should strive.
A concerted effort will be needed, however, to make sure SOC does not just become an empty buzzword. Vets need tools to assess patients, evidence to characterize the pros and cons of various treatment options, training and support in client communication and shared decision-making, and backing from veterinary organizations to support implementing SOC as a routine process.
More evidence also needs to be developed to elucidate the risk and benefits of an SOC approach and determine if this practice really improves greater accessibility and quality of veterinary care for patients. Like all apparently good ideas, the real the value of SOC needs to be demonstrated empirically.
For now, the rationale for the approach, and for building the necessary evidence, tools, and support systems to implement, seems strong, and the potential to improve the experience of all parties in the VCPR seems promising.
REFERENCES
1. Stull JW, Shelby JA, Bonnett BN, et al. Barriers and next steps to providing a spectrum of effective health care to companion animals. J Am Vet Med Assoc. 2018;253(11):1386-1389. doi:10.2460/javma.253.11.1386
2. Brown CR, Garrett LD, Gilles WK, et al. Spectrum of care: more than treatment options. J Am Vet Med Assoc. 2021;259(7):712-717. doi:10.2460/javma.259.7.712
3. Fingland RB, Stone LR, Read EK, Moore RM. Preparing veterinary students for excellence in general practice: building confidence and competence by focusing on spectrum of care. J Am Vet Med Assoc. 2021;259(5):463-470. doi:10.2460/javma.259.5.463
4. Alves JC, Santos A, Jorge P, Lavrador C, Carreira LM. Evaluation of Four Clinical Metrology Instruments for the Assessment of Osteoarthritis in Dogs. Anim Open Access J MDPI. 2022;12(20):2808. doi:10.3390/ani12202808
5. Schmutz A, Spofford N, Burghardt W, De Meyer G. Development and initial validation of a dog quality of life instrument. Sci Rep. 2022;12(1):12225. doi:10.1038/s41598-022-16315-y
6. Spitznagel MB, Carlson MD. Caregiver Burden and Veterinary Client Well-Being. Vet Clin North Am Small Anim Pract. 2019;49(3):431-444. doi:10.1016/j.cvsm.2019.01.008
7. Silva PTRF, Coura FM, Costa-Val AP. Caregiver Burden in Small Animal Clinics: A Comparative Analysis of Dermatological and Oncological Cases. Anim Open Access J MDPI. 2024;14(2):276. doi:10.3390/ani14020276
8. McKenzie B. Do it yourself or send for help? Considering specialty referral from a general practitioner perspective. J Am Vet Med Assoc. Published online January 3, 2024:1-6. doi:10.2460/javma.23.11.0612
9. Englar RE. The Gold Standard, Standards of Care, and Spectrum of Care. In: Low-Cost Veterinary Clinical Diagnostics. John Wiley & Sons, Ltd; 2023:1-8. doi:10.1002/9781119714521.ch1
10. McKenzie B. Evidence-based veterinary medicine: What is it and why does it matter? Equine Vet Educ. 2014;26(9):451-452. doi:10.1111/eve.12216
11. Cooper ES, Owens TJ, Chew DJ, Tony Buffington CA. A protocol for managing urethral obstruction in male cats without urethral catheterization. J Am Vet Med Assoc. 2010;237(11):1261-1266. doi:10.2460/javma.237.11.1261
12. Venn EC, Preisner K, Boscan PL, Twedt DC, Sullivan LA. Evaluation of an outpatient protocol in the treatment of canine parvoviral enteritis. J Vet Emerg Crit Care San Antonio Tex 2001. 2017;27(1):52-65. doi:10.1111/vec.12561
In the endless debates about the health effects of various approach to feeding our canine and feline companions, the subject of “processed foods” or “ultra-processed foods” comes up often. Generally, the argument is made that traditional commercial pet foods, including canned but most especially extruded dry foods (aka kibble), are “ultra-processed” and are functionally equivalent to potato chips and sliced lunch meat. Since the evidence is pretty consistent that convenience foods, packaged snack foods, and most “fast-foods” are associated with increased health risks in humans, the conclusion is that these traditional commercial foods must be unhealthy for our pets.
This is a superficially logical argument, but it is also convenient for proponents of various unscientific approaches to animal health because it fits nicely with the “appeal to nature fallacy” many of them are committed to. That is the idea that something which appears “natural” to them is healthier than something which seems artificial or unnatural.
This is, of course, an arbitrary and nearly meaningless aesthetic judgment, not a rational or scientific categorization. However, since the health risks of ultra-processed foods for humans, and the apparent equivalence of traditional pet diets with such foods, reinforce an existing bias, this is taken as a strong argument for alternative diets that present the appearance of being more “natural: and more like the less processed “whole foods” widely recommended for humans.
In these discussions, I have tried many times to point out a clear and gaping hole in this reasoning—just because commercial pet foods come in a bag or a can, doesn’t mean they are all the same or that they are equivalent to human snack foods. Potato chips, lunch meats, and fast foods are intentionally designed to be appealing, in terms of appearance, taste, sensory experience (e.g. mouth feel and smell), and low cost. They are not meant to be nutritionally substantive or “healthy” in any way.
This is quite different from pet foods, which are deliberately formulated to be nutritionally appropriate and to support long-term health. The “processing” is not the real problem with “processed foods,” it is the excesses of salt, calories, and harmful fats and the absence of micronutrients, fiber, and other dietary components that make such foods a health risk.
A recent research article and accompanying editorial make this point quite clearly.
Fang Z, Rossato SL, Hang D, Khandpur N, Wang K, Lo CH, Willett WC, Giovannucci EL, Song M. Association of ultra-processed food consumption with all cause and cause specific mortality: population based cohort study. BMJ. 2024 May 8;385:e078476.
This study evaluated diet and health outcomes in tens of thousands of North American healthcare workers followed for over thirty years. Here are some key findings:
All of this supports the existing evidence that UPF represent a health risk, but it also puts this in perspective. The risk is relatively small, much smaller than other known risk factors such as smoking, drinking excessively, or driving without a seat belt.
Even more telling, “this association was no longer apparent after overall diet quality was taken into account.” This means that there was no detectable difference between those who ate a lot or only a little UPF when the overall quality of the diet was accounted for. Again, the problem with UPF is not the “processing” but the unhealthy nutritional composition, and people who ate a generally healthy diet did not significantly increased their risk of dying if they included some UPF.
This point is further reinforced by the finding that the mortality risk difference was greater when alcohol was included as a UPF, and it was less when whole-grain bread products were included as a UPF. The health risks were affected by the nutritional quality and the other risk factors associated with a food (such as the toxic nature of ethyl alcohol) regardless of whether both items were equally “highly processed.”
The specific types of food most closely associated with increased mortality risks were meat products and sweetened beverages (though, interestingly, drinks sweetened with sugar had a slightly stronger negative effect that artificially sweetened, again undermining the “natural is better” argument).
The authors state their conclusion based quite clearly, and it reflects the point I have been making for years now:
Our data together suggest that dietary quality has a predominant influence on long term health, whereas the additional effect of food processing is likely to be limited.
Because the nutritional composition and overall diet quality is what matters, not the level of processing, the implications of this for diet recommendations is clear:
Limiting total ultra-processed food consumption may not have a substantial influence on premature death, whereas reducing consumption of certain ultra-processed food subgroups (for example, processed meat) can be beneficial.
The accompanying editorial expand son this point:
Not all ultra-processed food needs to be universally restricted and…careful deliberation is needed when considering whether to include recommendations about ultra-processed food in dietary guidelines. In countries where affordable, mass produced packaged wholegrain products such as breads are a recommended dietary staple and a major source of fibre, adding a sweeping statement in dietary guidelines about avoiding ultra-processed foods is not helpful.
As veterinary professionals and pet owners, these results reinforce the point that we should not obsess about the “processing” involved in the making of the pet foods we use. While inclusion of some fresh or whole foods may well turn out to have some health benefits, it is far more important that making sure our pets are eating complete and balanced diets that meet their nutritional needs, regardless of the form they take.
Over the years, I have reviewed the general evidence and some specific studies concerning vegetarian and vegan diets for dogs and cats. Despite the aggressive claims of some advocates for such diets (including some egregiously unscrupulous individuals), the actual evidence has not been extensive or definitive. My conclusions in previous posts have been that there is no clear evidence vegetarian or vegan diets have benefits for dogs and cats, and there is some real potential for harm, especially in cats:
Vegetarian Diets for Dogs & Cats, 2019
There is no evidence that vegetarian diets have health benefits for dogs and cats, and no real reason to believe they should be, based on the physiology and nutritional requirements of these species.
Dogs are omnivores shaped by domestication to be able to eat both plant and animal foods, and in theory they should be able to thrive on vegetarian or vegan diets. However, these diets must be carefully formulated, and many commercial vegetarian dog foods do not appear to be nutritionally adequate. There is also little reliable research evidence showing that dogs can remain healthy fed only a vegan diet.
Cats are clearly obligate carnivores with nutritional requirements that are unlikely to be effectively met by vegan diets. Such diets offer only risks and no benefits for cats and should be avoided.
Plant-based vs Meat-based Diets for Cats: Which is Healthier?, 2021
This study didn’t actually evaluate the effect of plant-based vs. meat-based diets on health or longevity in cats. What the study evaluated was the perceptions of owners about their cats’ diet and health. The difference is crucial.
All we can say is that owners who choose to feed a plant-based diet believe their cats are a healthier weight than owners who feed meat-based diets. Since plant-based diets are fed to a small minority of cats, the people who feed these diets must choose to do so based on pre-existing beliefs about their health value. Such individuals already believe these diets are healthier, and they are likely to see and report what is consistent with these beliefs, whether or not it is the reality of their cats’ condition.
Prospective, blinded, randomized feeding studies would be needed to allow any strong conclusions about whether or not plant-based diets are safe and healthy for cats.
Are Vegan Diets Healthier for Dogs & Cats?, 2022
The particular population of pet owners surveyed believes that feeding raw and plant-based diets are associated with better health in their pets. They also believe that their veterinarians think their pets are healthier (though whether these vets actually believe this is unknown)…Like previous studies relying on owner surveys and both conducted and funded by folks with strong a priori opinions about diet and health, this is a useful insight into such beliefs. It is not compelling or probative evidence for actual health effects of different feeding strategies.
Controlled studies with objective measures of outcome and more defined and verified feeding practices are required to draw any meaningful, actionable conclusions about the healthiest feeding strategy for our pets.
I am neither for nor against vegan diets for dogs, and I am even open to reversing my objection to feeding vegan to cats or raw diets to cats or dogs if strong evidence is generated that these are safe or beneficial practices.
I recently came across a systematic review of the literature that summarizes and assesses the available evidence concerning vegan diets for dogs and cats.
Domínguez-Oliva A, Mota-Rojas D, Semendric I, Whittaker AL. The Impact of Vegan Diets on Indicators of Health in Dogs and Cats: A Systematic Review. Veterinary Sciences. 2023; 10(1):52.
The data from review largely support my concerns about the lack of evidence, though the authors draw a somewhat more optimistic conclusion than I would:
In this review, we conducted a formal assessment of the evidence in the form of a systematic review. We found that there has been limited scientific study on the impact of vegan diets on cat and dog health. In addition, the studies that have been conducted tended to employ small sample sizes, with study designs which are considered less reliable in evidence-based practice. Whilst there have been several survey studies with larger sample sizes, these types of studies can be subject to selection bias based on the disposition of the respondents towards alternative diets, or since answers may relate to subjective concepts such as body condition. However, there is little evidence of adverse effects arising in dogs and cats on vegan diets. In addition, some of the evidence on adverse health impacts is contradicted in other studies. Additionally, there is some evidence of benefits, particularly arising from guardians’ perceptions of the diets. Given the lack of large population-based studies, a cautious approach is recommended.
The short version of this is that there aren’t many studies, and most have serious flaws or limitations, so strong conclusions either way aren’t justified.
No obviously horrible risks have shown up, but given that the studies which looked at actual health outcomes were few, short-term, and involved small numbers of animals on several very different diets, it would be a mistake to draw the general conclusion that vegan diets are safe.
Similarly, a few studies claim to show health benefits, but these are all based on owner surveys with groups of owners self-selected to include people already convinced that vegan diets are healthier. This kind of evidence, also frequently cited to support health claims for raw diets, is highly biased and tells us more about what believers in such diets expect to see than about the actual health effects on their pets.
Finally, some nutritionists have pointed out a significant problem with this kind of review– the idea that broad categories of diets (e.g. canned, kibble, raw, fresh, vegan, etc.) can be identified as beneficial or harmful doesn’t really make sense. The health impact of a diet depends on the specific composition, the ratio and availability of nutrients in the diet, and the nutritional needs of each individual. Vague generalizations, such as “vegan diets are healthy” or “kibble is unhealthy” are so broad as to be pretty meaningless, and they don’t help us decide what the best diet is for our specific pets. While some level of generalization is, of course, necessary and useful, these characterizations go too far to be accurate or helpful.
Bottom Line
There is very little research examining the health effects of vegetarian or vegan diets in dogs and cats. The existing evidence has significant limitations, which makes any firm conclusions impossible.
General theoretical arguments for why such diets should be healthy are not especially plausible nor convincing. It is likely that a properly formulated vegan diet could be adequate for many dogs, and possible for cats as well, but whether there are any benefits to feeding such diets, and whether these might outweigh potential risks, is not yet known.
Given this uncertainty, and the much better evidence for the appropriateness of cooked, meat-based diets, feeding dogs and cats a specific vegan diet is essentially a haphazard experiment, with as much or more potential for harm as for benefit.
Here are some low-quality recordings of a couple lectures I gave this year at the Western Veterinary Conference in Las Vegas.
Back in 2011, I first wrote about the issue of concerning whether dogs with cranial cruciate ligament (CCL) ruptures did better with surgery or with non-surgical management. My conclusion at that time was:
For most dogs under 15kg, conservative management (primarily restricted activity for 3-6 weeks, achieving and maintaining and appropriate body weight, and possibly physical therapy and pain medication) can achieve acceptable comfort and function. In larger dogs, significant arthritis is inevitable and dysfunction is extremely likely without surgical treatment.
In 2013, I write an update looking at an additional study , and concluded:
This study does provide some support for the contention that overweight, large-breed or giant-breed dogs have better long-term outcomes when treated with both surgery and non-surgical therapy rather than with non-surgical therapy alone. However, the limitations in these data are great enough that the case for preferring surgical intervention is not strong
Since that time, there has been some further research, but there has not been one single, definitive clinical study comparing surgery with other approaches for managing CCL disease. This is partly for ethical reasons. Since most vets believe surgery produces a better outcome, it is considered unethical to randomly assign dogs with CCL disease to getting surgery or getting a potentially inferior treatment.
A new study has attempted to use existing data on a large number of dogs, and some complex analytic techniques, to mimic such a study.
Camilla Pegram, Karla Diaz-Ordaz, Dave C. Brodbelt, Yu-Mei Chang, Anna Frykfors von Hekkel, Chieh-Hsi Wu, David B. Church, Dan G. O’Neill. Target Trial Emulation: Does surgical versus non-surgical management of cranial cruciate ligament rupture in dogs cause different outcomes? Preventive Veterinary Medicine. 2024; 226;106165.
I don’t have the expertise to evaluate the analytic approach in this study. The authors acknowledge many of the usual limitations to large retrospective analyses, but despite these issues, such studies are valuable, especially I the evidence-poor environment of veterinary medicine.
The results are pretty consistent in showing better outcomes in dogs treated surgically:
The current study shows that on average, surgical management leads to reduced lameness and analgesic prescription outcomes compared with non-surgical management.
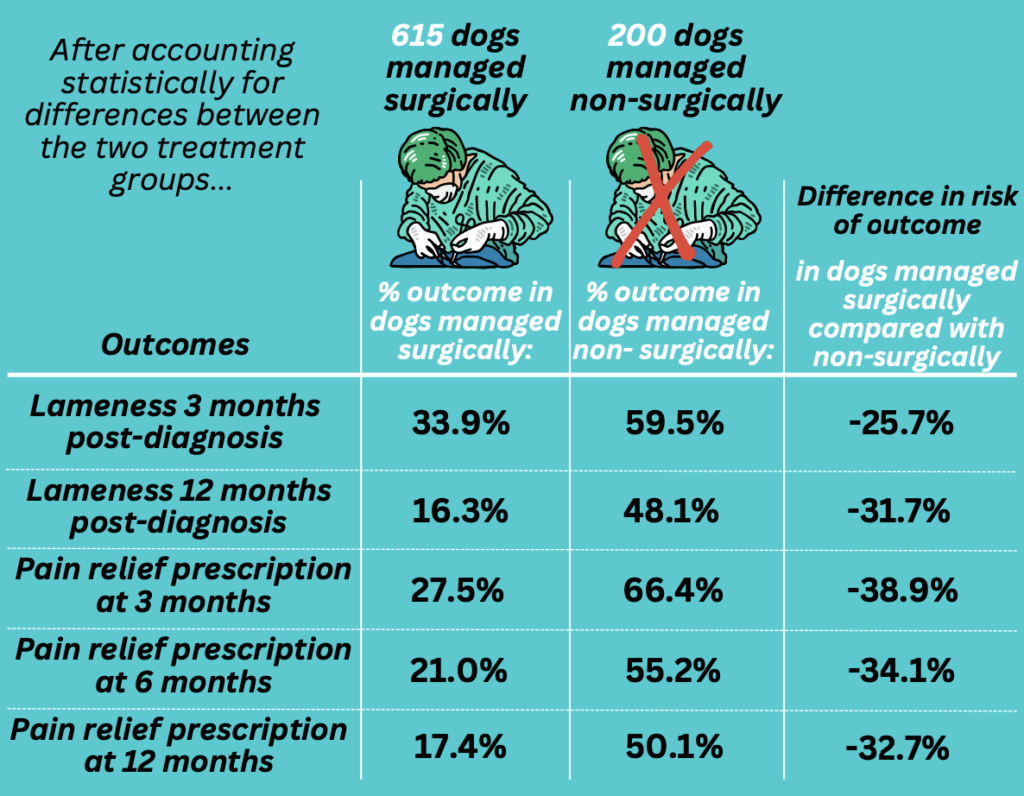
Interestingly, the study did not find any difference between large and small dogs. Both groups seemed to do better with surgery, which is a different finding than some previous research. The authors suggest this may be related to limited numbers of small dogs being treated, since they are less likely to develop CCL disease, so further work is needed to clarify the impact of size on the choice of treatment.
While there are always individual factors to integrate into any decision about the best management for a specific patient, this additional evidence tends to support the existing view that surgery probably produces better outcomes for dogs with CCL disease. While this is not the perfect definitive clinical trial, such a study is unlikely to occur. The evidence that we do have is pretty consistent, and it supports at least a moderate degree of confidence in recommending surgery for those patients in whom it is an option and who have no specific reasons to avoid surgical treatment.