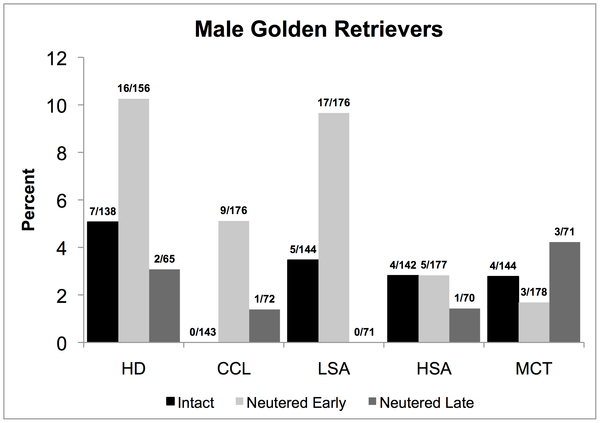
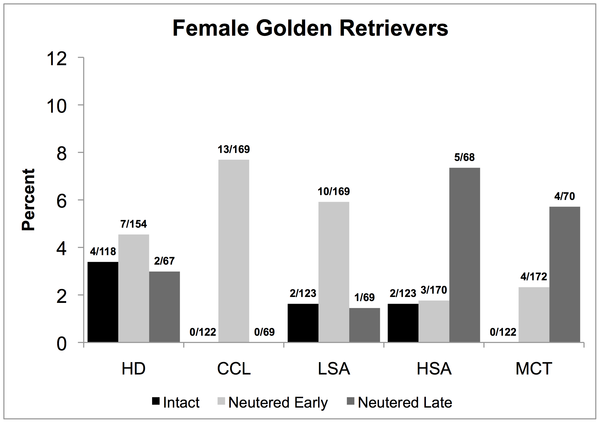
A perennially hot topic in human medicine is the risks and benefits of screening tests. Blood tests, imaging (like CT scans) and other diagnostic tests are usually seen by the general public as only beneficial. How could it be a bad thing to detect a disease, even before it is causing any symptoms? The reality, of course, is that such tests come with risks as well as benefits, like everything else in medicine.
I’ve written a bit about screening tests before, and I wrote an enthusiastic review of Gilbert Welch’s book Over-Diagnosed, which I believe should be required reading for anyone who is a healthcare provider or patient. The previously underappreciated dangers in inappropriate diagnostic tests have driven significant and sometimes controversial recommendations from the United States Preventative Services Task Force (USPSTF), the federal agency responsible for generating evidence-based guidelines concerning preventative medicine. And a broad coalition of medical specialty groups have formed an organization called Choosing Wisely which is dedicated to promoting sensible use of diagnostic tests by education physicians and the public about the risks and uncertainties, as well as the benefits, of such testing.
In general, the benefits of screening tests involve either allowing a disease to be treated at an earlier stage, even before the patient is aware of having it, and thereby improving the long-term outcome, or detecting risk factors for diseases which can be managed to reduce the chances of those diseases developing at all. For this to work, of course, the tests must screen for something which is treatable or preventable. A test which warns you in high school that you will die of some horrible disease in 30 or 40 years, and that there is nothing you can do about it, isn’t beneficial. In fact, it is more likely to do harm in that it generates anxiety and affects how you live your life in ways that don’t make you any healthier or happier.
Effective screening tests must also be accurate enough that they don’t miss a lot of cases of real disease or indicate disease is present in a lot of people when it really isn’t. No test is perfect, but most medical screening tests can be evaluated in people know to have, or not have, the condition tested for, which can give us an idea how accurate a particular test is. This generates the “sensitivity” and “specificity” numbers often cited to identify how reliable a test is.
For statistical reasons I won’t get into, these numbers can be misleading. Even a test which very rarely misidentifies someone as having a disease they don’t actually have will still misidentify a high proportion of people tested if the disease is really rare. So even the best tests aren’t very useful if we test people who are very unlikely to actually have the disease we are looking for. This is one reason why indiscriminate use of diagnostic tests is a not effective or reliable.
The devil, as always, is in the details, so it is difficult to generalize about when screening tests should or shouldn’t be used. For the most part, the guidelines in humans recommend using most screening tests only when there is some reason to suspect the disease one is looking for is present. If a person has typical symptoms, or is in a demographic group known to be frequently affected by the disease of interest, then a screening test might make sense.
Testing women for prostate cancer with a blood test such as the PSA, for example, is obviously ridiculous. The disease is never going to be present. And even with very good tests, false positives can show up, so what would a doctor do with such a positive test result? Most cases aren’t this clear cut, of course, but the same logic applies for all screening tests. The more likely the disease is to be present, the more reliable a positive test result is. And the less likely the disease is to be present, the more we can trust negative results. So choosing rationally who to test is a very important part of getting reliable results.
So what are the risks of screening test? Well, I’ve already touched on one—the unproductive anxiety associated with being misdiagnosed or with being correctly diagnosed with a disease you don’t have. Many people this hasn’t actually happened to will say they aren’t worried about this and would want to be tested even if this is a possible risk. However, people who have had the experience of a mistaken diagnosis, or a diagnosis they can’t do anything about, describe it as a terrible, life-altering experience. In veterinary medicine, this anxiety is unlikely to affect our patients directly. But there is no question it can profoundly affect their owners, as well as how their owners treat their pets, so this is still a risk to consider.
A more tangible risk of misdiagnosis is the danger and discomfort associated with unnecessary follow-up tests or treatment. Thousands of men have been seriously harmed by unnecessary testing and treatment for prostate cancer that they either never had or that would never have made them ill. This is one of the examples that has driven the more cautious and rational approach to screening tests in humans, and it is relevant to veterinary patients as well. If we diagnose disease that aren’t there, we are inevitably going to harm, and even kill, patients who would have been better off if we had not tested them in the first place. This risk has to be recognized and appropriate steps taken to minimize it, including using tests in a rational, evidence-based way.
And though it seems unpopular in America to acknowledge that it matters, the cost of unnecessary testing is a real issue. In human medicine, healthcare costs are a significant economic burden with widespread harm throughout the economy. And in veterinary medicine, where euthanasia is often chosen when money for further testing or treatment runs out, wasting money on unnecessary tests can easily lead to an inability to provide care that is actually needed.
As usual, the evidence concerning the risks and benefits of screening tests, and the attention given to the issue, are far less in veterinary medicine than in human medicine. However, there is some research evidence looking at screening tests in veterinary patients. Given the aggressive push from the AVMA for more preventative care, which is not a transparent, evidence-based effort and which in some ways appears driven as much by concern about revenue as concern about patient welfare, it is worthwhile to consider the issues involved in recommending screening tests for veterinary patients.
The most common and widely recommended screening tool is the annual physical examination. In human medicine, there is growing doubt about whether physical exams of apparently healthy people is useful or beneficial. There is almost nothing in the way of controlled research on the subject in veterinary medicine.
Certainly, every vet can think of examples of a patient whose owners were not aware of any problem but who turned out, after a good physical exam, to have a serious illness. We are most likely, of course, to remember those examples in which the illness was treatable, since happy endings that make us feel good about the work we do tend to stick in our minds. We may or may not remember cases in which the illness we identified couldn’t be treated or was treated in a way that did little to improve the well-being or longevity of the patient and perhaps even made it worse due to side effects of the treatment. And, of course, we can’t possibly remember those cases in which a client declined to accept the initial test or follow-up investigation and treatment and the disease never materialized. Our memory of individual cases is not, unfortunately, a very reliable way to decide whether physical exams of apparently health pets are beneficial to our patients or not. Availability bias, confirmation bias, cognitive dissonance, and many other cognitive quirks make such anecdotes deeply misleading.
One obvious difference between human and veterinary medicine is that people can tell their doctors about symptoms they experience which might suggest a disease even when there is no outward sign of one. Our pets have a much more limited ability to make known how they feel, and in fact may act8ively hide signs of illness from us. This supports the argument that physical exams, and other diagnostic tests, may be justified in apparently healthy pets even when they aren’t in apparently healthy humans. However, as reasonable as such an argument is, it remains unproven.
The other kind of diagnostic test frequently recommended is bloodwork. There is absolutely no consistency within the profession on what kind of screening blood tests should be done when, how often, and in which patients. It is common to recommend some kind of bloodwork before anaesthesia, and often on some regular basis in older pets, but there are few guidelines, those that do exist are based on opinion and experience rather than high-quality evidence, and individual veterinarians and hospitals vary widely in their recommendations.
There have been a couple of studies looking at how often abnormalities are detected on pre-operative bloodwork, and to a lesser extent whether these abnormalities affected the treatment the patient received. I will discuss a couple of these, which I think fairly represent the state of the evidence at this time.
Alef, M.; Praun, F. von; Oechtering, G. Is routine pre-anaesthetic haematological and biochemical screening justified in dogs? Veterinary Anaesthesia and Analgesia 2008 Vol. 35 No. 2 pp. 132-140
Abstract
Objectives: To determine if routine haematological and biochemical screening is of benefit in dogs requiring anaesthesia and to establish the most useful tests for pre-anaesthetic risk assessment. Animals: One thousand five hundred and thirty-seven client-owned dogs undergoing surgery at the University of Leipzig between January 2003 and April 2004.Materials and methods: After obtaining a standardized history and a physical examination, all dogs requiring anaesthesia were assigned to an ASA physical status group, their needs for pre-anaesthetic therapy determined and an anaesthetic protocol proposed. Haematological (haematocrit, red blood cell count, white blood cell count, platelet count and haemoglobin concentration) and serum biochemistry tests (plasma urea, creatinine, glucose, total protein, sodium and potassium concentration; serum alanine aminotransferase, alkaline phosphatase and lipase activity) were then performed in all animals. The results of these were then used to: (1) re-define each dog’s ASA physical status; (2) determine any altered requirement for pre-anaesthetic therapy; (3) re-determine the suitability of the dog to undergo surgery; and (4) re-examine the suitability of the original proposed anaesthetic protocol.
Results: The history and clinical examination in 1293 out of 1537 dogs (84.1%) revealed that haematological and biochemical tests would have been considered unnecessary under normal conditions. Of these, 63.9% were categorized as ASA 1, 28.5% as ASA 2, and 7.6% at higher risk. In some dogs, screening tests showed abnormal results: 16.7% of 1293 dogs had abnormal plasma urea levels, with 5.9% of values above the reference range. However, only 104 dogs (8%) would have been re-categorized at a higher physical status category had the laboratory results been available. Additional screening data indicated that surgery would have been postponed in 10 dogs (0.8%) additional pre-anaesthetic therapy would have been provided in 19 animals (1.5%) and the anaesthetic protocol altered in two dogs (0.2%).
Conclusion: The changes revealed by pre-operative screening were usually of little clinical relevance and did not prompt major changes to the anaesthetic technique.
Clinical relevance: In dogs, pre-anaesthetic laboratory examination is unlikely to yield additional important information if no potential problems are identified in the history and on physical examination.
This study fairly clearly shows that only a very small number of dogs presenting for surgery with no reason to suspect illness actually show abnormalities on screening bloodwork, and these abnormalities almost never influence the care these dogs are given. Of course, the question remains open whether testing thousands of dogs to identify relevant problems in maybe 2% is appropriate. Do the benefits for a few individuals (whatever those are, since the study didn’t actually look at the outcome of the procedure) outweigh the costs, the risk of misdiagnosis, harm from unnecessary follow-up testing or unnecessary deferral of needed surgical procedures, and the stress to the owners of dogs who are misdiagnosed?
Another similar study looked at a population for which there is a reasonable rationale to performing pre-operative screening; geriatric dogs.
K E Joubert. Pre-anaesthetic screening of geriatric dogs. J S Afr Vet Assoc. March 2007;78(1):31-5.
Introduction: Pre-anaesthetic screening has been advocated as a valuable tool for improving anaesthetic safety and determining anaesthetic risk. This study was done determine whether pre-anaesthetic screening result in cancellation of anaesthesia and the diagnosis of new clinical conditions in geriatric dogs.
Methods: One hundred and one dogs older than 7 years of age provided informed owner consent were included in the study. Each dog was weighed, and its temperature, pulse and respiration recorded. An abdominal palpation, examination of the mouth, including capillary refill time and mucous membranes, auscultation, body condition and habitus was performed and assessed. A cephalic catheter was placed and blood drawn for pre-anaesthetic testing. A micro-haematocrit tube was filled and the packed cell volume determined. The blood placed was in a test tube, centrifuged and then analysed on an in-house blood analyser. Alkaline phosphatase, alanine transferase, urea, creatinine, glucose and total protein were determined. A urine sample was then obtained by cystocentesis, catheterisation or free-flow for analysis. The urine specific gravity was determined with a refractometer. A small quantity of urine was then placed on a dip stick. Any new diagnoses made during the pre-anaesthetic screening were recorded.
Results: The average age of the dogs was 10.99 +/- 2.44 years and the weight was 19.64 +/- 15.78 kg. There were 13 dogs with pre-existing medical conditions. A total of 30 new diagnoses were made on the basis of the pre-anaesthetic screening. The most common conditions were neoplasia, chronic kidney disease and Cushing’s disease. Of the 30 patients with a new diagnosis, 13 did not undergo anaesthesia as result of the new diagnosis.
Conclusions: From this study it can be concluded that screening of geriatric patients is important and that sub-clinical disease could be present in nearly 30 % of these patients. The value of screening before anaesthesia is perhaps more questionable in terms of anaesthetic practice but it is an appropriate time to perform such an evaluation. The value of pre-anaesthetic screening in veterinary anaesthesia still needs to be evaluated in terms of appropriate outcome variables.
This study appears to find more real and meaningful abnormalities, as one would expect in an older population. However, the details still showed that age did not reliably predict which dogs would have bloodwork abnormalities:
The effect of age and/or breed on the choice of pre-anaesthetic laboratory testing was not fully elucidated in the current study. However, preliminary results show only statistically significant differences (p < 0.05) in platelet count and ALT activity in dogs over 10 years of age. No consistent differences could be found between age groups (<2, 2–7, 7–10 and over 10 years) for the plasma concentrations of glucose, urea and lipase activity. Young dogs (<2 years) showed statistically significant but only slight differences to other age groups in total protein and sodium concentration.
Furthermore, there was no detectable difference in the chances of a pre-operative abnormality in dogs that did or did not have complications during surgery, so it is questionable whether the abnormalities that were found actually helped prevent problems from surgery (though this might have been affected by the deferral of surgery in dogs with some detected abnormalities):
Laboratory test results were within the reference range or interpreted as being clinically irrelevant in 21 of 25 dogs experiencing complications. Relevant laboratory findings were found in only four. The incidence of adverse incidents was 3.8% in dogs with ‘abnormal’ laboratory results and 1.8% in animals with ‘normal’ values. Because of the limited number of complications, no statistical difference could be shown between groups.
Certainly, more research is needed on this topic. However, the existing research suggests that routine pre-operative bloodwork is likely to benefit only a very few patients, so the questions about whether this justifies the costs and risks is still not easy to answer.
In human medicine, guidelines for pre-anesthetic screening are more conservative that what is routinely done by veterinarians:
The guidelines from the American Society of Anesthesiology Task Force on Pre-anesthetic Evaluation state the following:
Routine Preoperative Testing
• Preoperative tests should not be ordered routinely.
• Preoperative tests may be ordered, required, or performed on a selective basis for purposes of guiding or optimizing perioperative management.
• The indications for such testing should be documented and based on information obtained from medical records, patient interview, physical examination, and type and invasiveness of the planned procedure.
Preanesthesia Hemoglobin or Hematocrit
• Routine hemoglobin or hematocrit is not indicated.
• Clinical characteristics to consider as indications for hemoglobin or hematocrit include type and invasiveness of procedure, patients with liver disease, extremes of age, and history of anemia, bleeding, and other hematologic disorders.• Preanesthesia Serum Chemistries (i.e., Potassium, Glucose, Sodium, Renal and Liver Function Studies)
• Clinical characteristics to consider before ordering preanesthesia serum chemistries include likely perioperative therapies, endocrine disorders, risk of renal and liver dysfunction, and use of certain medications or alternative therapies.
• The Task Force recognizes that laboratory values may differ from normal values at extremes of age.
• Preanesthesia Urinalysis • Urinalysis is not indicated except for specific procedures (e.g., prosthesis implantation, urologic procedures) or when urinary tract symptoms are present.
The general policy in humans is to recommend specific tests based on specific indicators of risk for each individual patient, not to routinely screen everybody who is going to undergo anaesthesia.
A recent paper in the Journal of Feline Medicine and Surgery found a relatively high incidence of common problems (dental disease, kidney disease) etc. in cat over 10 years of age, which would support screening for these disorders in this population. However, this was a small study, and the population was different in some significant ways from those in other places (for example having a much higher incidence of FIV infection that is typical in the U.S.), so we must be cautious about generalizing these results.
I think a rational approach in veterinary medicine is to screen those patients which there are reasons to suspect may have relevant abnormalities, such as animals with clinical symptoms, older animals, animals with known pre-existing conditions, etc. In the absence of better evidence, the only firm conclusion we can make is that firm conclusions are probably not justified. Anyone who says that pre-operative screening or annual examinations of young, apparently healthy pets is mandatory and that not doing it is malpractice is expressing only their opinion, not a conclusion based on reliable scientific evidence. And anyone who states that such screening is worthless is also going beyond the evidence.
Decisions about screening tests in veterinary medicine are, like so many decisions in our business, as much about the values and risk tolerance of individual doctors and owners as about what can be demonstrated to be the best practice. The major deficiency at this point seems to me to be a failure of veterinarians to recognize that screening has costs and risks that should be considered as well as benefits. True informed consent requires that we make our clients aware of the concerns that have emerged in human medicine over irrational or indiscriminate use of screening tests, and of the limited evidence to support screening asymptomatic pets. Clients should be helped to make informed decisions which consider both the possible benefits of screening and also the costs and risks for them and their pets.